Chapter 4: The Basic Findings In Instrumental/Operant
Conditioning(1)
Overview: This chapter is arranged in four major
sections.
The first presents the background to Instrumental Conditioning,
covering the early work by Watson and Thorndike, the
basic
findings, what they thought was going on, and what some of the standard
paradigms for Instrumental Conditioning are. The second presents many
of
the basic principles that determine when conditioning will
occur,
and whether it will be excitatory or inhibitory. The
third
discusses important exceptions to these principles, and
examines
the complex interactions that can arise. Finally, the fourth
section
briefly examines several alternative accounts of the type of an
association that can form. Several additional accounts of learning are
introduced here; most notably, Tolman's Cognitive Expectancy
Approach
and Skinner's Radical Behaviorism. This section closes by
examining
some of the interrelationships between Instrumental and
Classical
Conditioning.
I. Introduction To Instrumental Conditioning
We now shift from the topic
of classical conditioning to that of instrumental (or as Skinner
terms it, operant) conditioning. This topic will prove
a
bit more complex in its findings. As you will see, however, many of the
ideas that were important in classical conditioning will prove relevant
here. Indeed, there has long been a debate over whether classical and
operant
conditioning ought to be regarded as truly different forms of
learning.
They appear to differ in the sense that classical conditioning
generally
involves the presence of reflex actions, whereas instrumental
conditioning
generally involves modifications of voluntary behavior
contingent
on presence of reinforcers or punishers. Whether that
is
a sufficient reason to distinguish them is arguable, as we will see
later.
My sense of the field today is that most theorists would like to see
similar
theories explain the results in both. Thus, it will not surprise you,
for
example, that a modified version of the Rescorla-Wagner model has also
been proposed for instrumental conditioning.
Let's start with some
historical
background.
A. Background: Two Early Views Of Instrumental Conditioning
We will look at two quite
different
claims about the nature of instrumental conditioning. One comes from Watson,
the author of the 1913 behaviorist manifesto, Psychology as the
behaviorist
views it, and the second comes from Thorndike, who can
probably
safely be credited with conducting the first truly sophisticated and
careful
observations of complex animal learning. Their accounts differ in ways
that prefigured an important debate about what was needed for learning
to occur.
First, however, let us
distinguish
instrumental conditioning from classical conditioning. In instrumental
conditioning, an animal makes one of a number of possible
responses
in the presence of some stimulus complex or context. That response may
lead to some outcome. We typically define learning in this circumstance
as an alteration in some observed characteristic of the response such
as
its frequency, latency, or amplitude. We will revisit this definition
in
more detail later, once we have examined several theories of what gets
acquired, and why. For now, we can talk about instrumental conditioning
as the type of learning involved in navigating a maze, choosing the
correct
one of several doors to run to, or even performing some response that
will
be successful in avoiding a future shock. In instrumental conditioning,
new responses may be taught that differ from any reflexive
response
already in the animal's behavioral repertoire.
Watson: Contiguity of S & R
As you know from Chapter 1,
Watson attempted to redefine the field of psychology in response to
then-current
mentalism. We have looked at several of the assumptions he brought
along.
Basically, he was an extreme environmentalist who believed that most --
if not all -- of our actions were under the learned control of
associations.
Based on his knowledge of work being done by people like Pavlov, Watson
certainly believed that living things were born with a repertoire of reflexes.
However, association quickly acquired control of previously reflexive
responses,
and indeed helped modify those responses to create new responses. Some
idea of Watson's radicalism on this point may be gathered from a very
famous
quote (1926, p. 10):
Give me a dozen healthy infants, well-formed, and my own
specified world to bring them up in and I'll guarantee to take any one
at random and train him to become any type of specialist I might select
-- doctor, lawyer, artist, merchant-chief and, yes, even beggar-man and
thief, regardless of his talents, penchants, tendencies, abilities,
vocations,
and race of his ancestors.
This was radical in at least two related ways. First, from a scientific
perspective, it clearly denied the relevance of genetic or inherited
influences
on current behavior. And second, from a social perspective, it was
about
as different a position as one could expect to find from the
then-prevailing
attitudes about race and class.
In any case, Watson's
primary
idea was that an association could form between a stimulus and a
response
(in addition to the type of association found in classical
conditioning).
But he was a strict contiguity theorist on the issue of S-R
associations:
A response made in the presence of a stimulus might associate with it,
and under certain circumstances, would be likely to be seen
when
that stimulus recurred. Those circumstances were defined by essentially
two principles. The first, a principle of frequency,
stated
that the association strengthened each time the response was made to
the
stimulus, so that all things being equal, a frequent response
was
much more likely to be emitted by the animal than a less frequent
response.
In addition, however, there was a principle of recency: All
things
being equal, a recent response was more likely to be emitted
than
a less recent response.
What you should
particularly
note about the brief description of Watson's system above is the
complete
lack of any reference to a reinforcer, a perhaps surprising
omission
to students who have been introduced to the idea that
instrumental/operant
conditioning is in large part about the effects of rewards and
punishments.
That wasn't so for Watson, and it has not always been so for later
theorists
as widely divergent as Guthrie and Tolman (see below
and
in the next chapter). But on a preliminary and casual analysis of
classical
conditioning, the notion of a reward or punishment does not seem
greatly
relevant in discussing whether the association forms. (Nevertheless,
some
theorists refer to the UCS as a reinforcer on a broad
definition
that a reinforcer is what makes a response more likely; presence of the
UCS, whether in excitatory-appetitive or excitatory-aversive
conditioning,
certainly accomplishes that!) Why, then, ought we to include it in
instrumental
conditioning?
And even though Watson
talked
about associations between stimuli and responses, he also allowed for
the
possibility of associations between responses themselves. Thus,
in the case of animals learning to run a maze, the analysis of what is
going on will involve a complex series of muscle movements
involving
motor responses. (Much of our behavior is complex, rather than the
execution
of simple responses to individual stimulus triggers.) Rather than talk
about external stimuli controlling each succeeding muscle movement that
gets the animal from Point A to Point B in the maze, Watson claimed
that
a chain of responses could be linked together that would be
initially
set off by an external stimulus. Of course, to the extent that any
response
also involves internal stimulation, one could still analyze
chains
in terms of stimulus-response links, so that each muscle movement in
the
chain serves as the response to the previous movement, and the
stimulus
for the next.
We have already discussed
in Chapter 1 Watson's insistence that thinking could be reduced
to subvocal speech. He also conducted experiments in emotional
conditioning. In a famous study with Rayner, Watson
conditioned
a young child, Little Albert, to be afraid of a white rat.
Every
time Albert played (apparently happily, at first) with the rat, an
experimenter
would creep up behind Albert and strike a metal bar, making a loud
clanging
noise that frightened Albert and caused him to cry. After several such
occasions (six, in fact), Albert started to cry at the sight of the
rat.
Note how this could be analyzed from the point of view of classical
conditioning:
The noise caused the apparent emotional response of fear, whereas the
rat
served as the CS.
Given what you know of
Watson's
views on mentalism, you may be somewhat surprised to discover him
talking
about the topic of emotions. However, for Watson, emotions were not
underlying
mentalistic events, but rather, the behavioral components (the
crying,
the whimpering, the shaking, etc.) observed in reaction to certain
stimuli.
Thus, Watson maintained a perfect consistency with respect to his
position
that positivism required dealing strictly with a behavioral
level.
Perhaps that is why he did not talk about reinforcers. Thorndike,
a contemporary of Watson's, was developing a theory of learning based
on
reinforcers, and although he defined them in a sufficiently
behavioristic
fashion, he was nevertheless attacked by others for apparently sneaking
mentalistic terms back into hard-nosed scientific psychology.
To reiterate a point I
made
earlier, later behaviorists have on occasion adopted a strict
contiguity
approach to learning. Most notable among these as a successor to Watson
was Guthrie, whose principle of conditioning stated (1952 , p.
23):
A combination of stimuli which has accompanied a
movement
will on its recurrence tend to be followed by that movement. Note
that nothing is here said about...reinforcement or pleasant effects.
As we will see later, such approaches were in part a reaction to work
by
Tolman and his colleagues suggesting that learning could occur in the
absence
of rewards or punishers. The question that faces such theorists then
becomes
one of explaining how and why rewards and punishers seem to influence
the
course of learning.
Thorndike & Puzzleboxes: Reinforcement-Based Learning
Rewards and punishers, in
contrast,
played a pivotal role in the work of Thorndike, who is often credited
with
founding the field of instrumental conditioning. Thorndike published a
monograph in 1898 on his studies with animals such as cats. He set up
an
experimental apparatus termed a puzzle box: a cage in which the
animal was placed, and which could be escaped through the performance
of
a simple response such as pulling on a rope attached to a door. These
studies
really involved the first careful, detailed observations of what
animals
in general learned, as opposed to anecdotal stories collected of
amazing
things animals did that obviously proved their intelligence.
(Television
still plays into that sort of approach, needless to say!)
Thorndike asked a very
simple
question: Would escape from a puzzle box exhibit any signs of intelligence?
Would it display evidence of insight, in which the animal would
be able to glance about its environment, understand that the rope was
attached
to the door, and realize that it needed only to pull on the rope to get
out? To answer this question, Thorndike repeatedly placed animals in
the
same puzzle box, and measured how long it took them to escape. And what
he found was that the time to escape decreased only gradually.
By
the end of the experiment, after 20 or so trials, cats would easily
leave
the box by performing the appropriate response as soon as they were
placed
in it. But, their history clearly demonstrated that this had to have
been
a learned response. In particular, Thorndike pointed out that an animal
making the correct response on a given trial early in training would
not necessarily choose that same response as its first response on the
next trial. So, rather than insight, he concluded that learning
involved
trial-and-error.
Trial-and-error refers to
the gradual accumulation of correct responses through a slow
process
of trying out all sorts of possibilities, and slowly weeding out the
ones
that do not work. As did Watson, Thorndike thought animals were
acquiring
associations between stimulus configurations (such as the puzzle box)
and
certain responses. But unlike Watson, he claimed that an additional
factor
was important in the acquisition of these associations: They would
depend
on the outcome of the animal's actions. This involved a
principle
Thorndike termed the Law of Effect. Put briefly, this law
claimed
that an association between a stimulus and a response would strengthen
if the response were followed by a satisfactory state of affairs,
and would weaken if the response were followed by an unsatisfactory
state of affairs. Thus, Thorndike deliberately included Bentham's
notion of hedonistic value as a principle governing the
formation
of an association, in contrast to Watson. Rather than being a simple
contiguity
theory, this was a reinforcement theory: In modern terms,
learning
of an association will occur when there is a reinforcer following a
response.
There are, of course, a
number
of interpretations available to account for how a reinforcer might
operate
according to the law of effect. One of the first to come to most
people's
minds is a teleological or purposive explanation: The
animal
performs a response because it desires the outcome. But of course, desiring
an outcome is a mental state that involves an object not present at the
time the animal is performing the response. That type of an explanation
would violate the positivist program Watson insisted everyone follow.
Thus,
as an alternative, we might propose that a positive outcome has an automatic
effect of strengthening the association: The animal does not perform
the
response because it wants the outcome, but rather because the response
is strongly associated to the stimulus that is present.
Here is what Thorndike
actually
said regarding satisfying and unsatisfying states (1913, p. 2):
By a satisfying state of affairs is meant one which the
animal does nothing to avoid, often doing things which maintain or
renew
it. By an annoying state of affairs is meant one which the animal does
nothing to preserve, often doing things which put an end to it.
Although he was accused of using hopelessly mentalistic terms in
describing
learning as depending on satisfactory or unsatisfactory states, his
actual
definition provided a clear behavioral test for determining when one or
the other state was present. In that sense, it ought to have troubled
people
no more than Watson's use of the term "emotional."
Note too that Thorndike
did
not include the outcome in the association. As we will see,
other
theorists have claimed that associations to the outcome may also form,
so that we can have S-R associations, R-O associations,
and
even S-O associations. To anticipate how such a model might
differ
from Thorndike's, a strong S-R association may exist despite a
highly
unpleasant or unsatisfying outcome: The presence of an R-O
association
in that event may serve to inhibit the R excited by presence of
an associated stimulus.
Thorndike also proposed
another
principle, the Law of Exercise (sometimes called the Law of
Use).
This was essentially a principle of practice, somewhat similar to
Watson's
notion of frequency: An association would strengthen if
practiced.
Both laws were revised in his later work: the Law of Effect was
essentially restricted to satisfactory outcomes, and the Law of Use
was modified to include outcomes rather than simple exercise.
Thorndike also spoke of
the
value of different satisfactory states, so that strong
satisfiers
would do a better job of strengthening an association than weak
satisfiers.
And as an interesting historical footnote, he actually contradicted one
of the major principles of strict contiguity by proposing an early
version
of belongingness by which some things would be more likely to
associate
together than others.
In some sense, Skinner
may be regarded as Thorndike's intellectual successor. Skinner proposed
similar ideas involving the law of reinforcement and the law
of punishment. According to Skinner, a reinforcer was any
event
that, following a response, made that response more likely,
whereas
a punisher was any event that had the opposite effect. To try
to
identify reinforcers and punishers in a way that wasn't completely
circular
(and also wasn't mentalistic), Skinner imposed a condition of transituationality:
A reinforcer or punisher, once identified in terms of its effects on one
response, also has to be shown capable of having a similar effect in other
situations, on other responses. Otherwise, we find
ourselves
defining a response as that which, when followed by a reinforcer,
increases
in frequency. And that type of definition, of course, reciprocally
defines
responses and reinforcers in terms of one another in an uninteresting,
circular fashion.
With this as background,
let us look at some of the basic findings in instrumental conditioning.
B. Some Basic Findings
Generalization, Discrimination, & Contrasts
Many of the basic findings
will
prove familiar, although there will also be some additional results of
interest. But in any case, as was true of classical conditioning, we
obtain
generalization, discrimination, and contrasts.
The usual procedure for
obtaining
generalization involves pairing a response with an outcome in the
presence
of a specific stimulus, and then presenting other stimuli to see
whether
there is a similar response to them. As outcomes may be of two sorts
(reinforcers
and punishers), we may obtain two different types of generalization
gradients.
The gradient associated with use of a reinforcer is termed the gradient
of excitation, whereas the gradient associated with use of a
punisher
is termed the gradient of inhibition. In an excitatory
gradient,
we look for responding to novel stimuli that is above the background
or baseline or operant level; and in a gradient
of
inhibition, we look for responding below normal.
Typically, when a
response
has been reinforced in the presence of the stimulus, that stimulus is
referred
to as S+. Similarly, when the response has been punished, the
stimulus
is referred to as S-. Watson and Rayner used an S-
with Little Albert. In their work, they also reported obtaining
generalization:
Albert developed fear reactions to other stimuli (such as rabbits and
coats)
involving the features white and fur. Although they had
planned
on reversing the fear conditioning, Albert's mother removed him from
the
daycare where they were doing their experiments.
A good example of a
gradient
of excitation may be found in the work of Guttman and Kalish.
They
took four different groups of pigeons and trained them to peck at a
colored
key. The key differed in color for the four groups (530, 550, 580, or
600
nanometers). Then, in a generalization test, Guttman and Kalish
presented
a series of 11 colors, one at a time, and simply counted the number of
pecks per 6 minute period that each color received. These colors
included
the original (the S+), 5 colors above the S+ in
wavelength,
and 5 colors below. Their results appear in Figure 1.
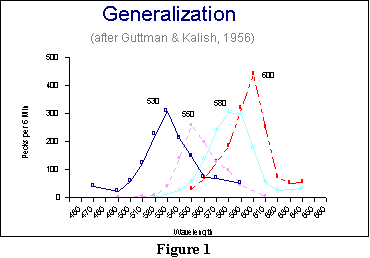 Several features of these results should be noted. First, the stimulus
that received the most pecks for each group was S+:
That
is where the peak of each generalization gradient may be found.
(As you will see later, this need not always be the case. Certain
experiences
such as discrimination training may alter the peak and shape of a
generalization
gradient.) Second, there was a relatively smooth drop-off of
responding
as the wavelengths of the stimuli increasingly differed from the S+.
And finally, the curves were symmetric: The left-hand side of
each
curve looked approximately like the right-hand side.
Several features of these results should be noted. First, the stimulus
that received the most pecks for each group was S+:
That
is where the peak of each generalization gradient may be found.
(As you will see later, this need not always be the case. Certain
experiences
such as discrimination training may alter the peak and shape of a
generalization
gradient.) Second, there was a relatively smooth drop-off of
responding
as the wavelengths of the stimuli increasingly differed from the S+.
And finally, the curves were symmetric: The left-hand side of
each
curve looked approximately like the right-hand side.
Similar features may be
found
in a gradient of inhibition. Rather than look for a peak,
however,
we search for a valley representing the lowest level of
responding.
Here, as the stimuli increasingly differ, we ought to find increasing recovery
of responding. Thus, a gradient of inhibition looks a bit like an
upside
down gradient of excitation. In each, the idea is that similarity
of
stimuli maps into similarity of responses.
As was true of classical
conditioning, there will be occasions in which we want to train an
animal
to treat apparently similar stimuli as if they were different. As a
parent,
you might think there to be good reason to train a child to fear rats
without
desiring that such reactions extend also to rabbits or cats. The
standard
technique for teaching a discrimination in instrumental/operant
conditioning
will prove similar to that introduced in classical conditioning: We
present
the outcome whenever the organism makes the response in the presence of
one stimulus, but not in the presence of another. To introduce
technical
terms, the stimulus that signals an effective response
(effective
in the sense of producing an outcome) is called the discriminative
stimulus
or discriminative cue (SD). The stimulus that
should come to signal an ineffective response is normally
represented
with a delta symbol. As I am posting this to the web where delta
symbols
are a bit tricky to insert into normal text, I will adopt the practice
of using S+ and S- in this situation, as well.
There are other
techniques
to train a discrimination, as you will see in a later chapter. Rather
than
associate one stimulus with no outcome, we can associate it with the
need
for a different response. Thus, perhaps the animal will need to
turn to the left for food when a red light is present, but will need to
turn to the right for food when an orange light appears. Such a
technique
is referred to as choice discrimination. Alternatively, we
might
slowly introduce the second stimulus into the animal's environment,
presenting
it initially at very low levels of intensity. If the intensity is
slowly
increased, we may find that our animal has never responded to it, thus
foregoing generalization. (You should be wondering whether there is
something
like latent inhibition going on with this procedure!) This technique is
referred to as errorless discrimination. Each technique
appears
to have different effects on the generalization gradient. In
particular,
the standard technique using S+ and S- seems to cause
the
peak to move away from S+, and to the side opposite S-,
a
phenomenon referred to as peak shift. Moreover, peak shift is
typically
associated with a gradient that is no longer symmetrical: The
gradient
appears to be 'bunched up' on the S- side.
Finally, we may also note
the existence of contrast effects associated with these
phenomena.
There are several types of contrasts found in instrumental
conditioning.
One that accompanies peak shift is termed behavioral contrast. Hanson,
for example, compared discrimination learning and non-discrimination
learning
groups. The discrimination-learning group displayed a peak shift. Their
responding to the S+ dropped off considerably. But the
responding
to the stimulus that was the new peak increased dramatically. This
group
displayed about twice as many responses to this untrained
stimulus
compared to the control group that did not have discrimination
training.
Thus, in a behavioral contrast, responding occurs to a novel
stimulus
at a greater level than would be expected on the basis of simple
generalization.
Inhibition In Extinction & Punishment
Extinction in
instrumental
conditioning will involve essentially the same process of decoupling
that
we saw in classical conditioning. That is, we generally remove the
outcome
after which we ought to see the response return to normal or baseline
levels (sometimes referred to as operant levels). As is the
case
for acquisition (and for classical conditioning), the learning
curve
for extinction typically involves diminishing returns. How long
it takes a response to return to pre-learning (baseline) levels is
referred
to as its resistance to extinction: Responses that quickly
return
to baseline levels have a low resistance to extinction, whereas
those that take a long time to return to baseline have a high
resistance.
Resistance to extinction will depend on a number of factors
including
the value of the outcome (see below), the energy
required
to make the response (more physically demanding responses generally
have
lower resistance), and the past history of training (responses
learned
under partial reinforcement conditions generally have greater
resistance
to extinction than those acquired under continuous reinforcement
conditions:
see below and the later chapter on partial reinforcement and
extinction).
As was true in classical
conditioning, extinction in instrumental conditioning is viewed as a
type
of inhibitory learning. Following extinction, we obtain similar
patterns
of spontaneous recovery and relearning that we did with
classical
conditioning: An extinguished response tends to recur after a while
(spontaneous
recovery), arguing against any claim that the association acquired
during
the acquisition phase had actually been destroyed or forgotten.
Similarly,
pairing the extinguished response with the outcome results in much
faster
acquisition (relearning), another argument suggesting extinction does
not
destroy the original learning.
Moreover, a stimulus
associated
with extinction appears to act as an aversive stimulus for the animal,
suggesting some degree of inhibition. Daly, for example, found
that
rats would learn to escape from a place where they had earlier expected
a reinforcer. When the reward was no longer available, the contextual
cues
associated with that location were sufficient to motivate the animal to
avoid them by learning some new response getting it out of that
situation.
Complicating the picture
somewhat is the fact that an outcome may be a punisher. Punishment, of
course, often suppresses a response. There has been an argument
extending
as far back as Thorndike concerning the effectiveness of punishment.
Many
people have reported that punishers seem to have, at best, temporary
suppressive
effects on on-going behavior. However, that issue appears to involve
the
intensity of the punisher. There is now plenty of evidence that highly
aversive punishers may have long-lasting effects. According to Bolles,
stimuli present when a punisher occurs may become conditioned danger
signals that will tend to interfere with on-going behavior by
activating
the animal's instinctive defenses (SSDRs: species-specific defense
reactions).
Rats, for example, will run, freeze, or fight. So, in this case, a
conditioned
suppression-like reaction may occur because one of these responses will
be incompatible with other excitatory responses such as pressing a
lever
for food.
In the case of a punished
response, of course, extinction of that response by no longer
associating
it with an aversive outcome ought to inhibit the stimulus's ability to
act as a danger signal triggering an SSDR. Inhibition of aversion in
this
case means seeing less aversion.
One more point while we
are
(briefly) on the subject of punishment: One of the difficulties
theorists
have had with the effects of punishment (and with positing a general
principle
that punished responses decrease in frequency) may be seen from a study
by Brown, Martin, and Morrow. They taught rats to run an
alleyway
to escape shock. Basically, the alleyway was electrified, so the
animals
needed to run to the goal box (the only non-electrified, safe
portion
of the alleyway). When the shock was turned off, there was fairly quick
extinction of running.
However, two other groups
of rats were also put through an extinction procedure. For one of these
groups, the shock was also turned off in the start box, so that
they would actually be punishing themselves for venturing out of the
start
area. The other group had the final 2 feet (of a 10-foot alleyway)
electrified,
so that they would be punished by trying to get to the goal box.
Curiously
enough, these two groups did not extinguish anywhere near as rapidly:
By
the 6th day of extinction, they were still running to the
goal
box, giving themselves needless shocks. Thus, punishment sometimes can
actually prolong the response being punished. This effect is
called
vicious circle behavior.
Within the framework of a
model such as Bolles's theory, a finding like that of Brown
et
al. may be accounted for in terms of shock continuing to trigger
the
rat's running SSDR. It is also possible that vicious circle
behavior
may ensue because of multiple mechanisms. Thus, in another
experiment,
Badia and Culbertson set up a situation in which shock could be
signaled or unsignaled. In signaled shock, a
stimulus
will come on slightly before the shock. In this study, they allowed
rats
to learn a response whose only reinforcer involved shocks being
signaled.
Their animals acquired the response. Moreover, Badia, Culbertson,
and
Harsh found that given a choice between unsignaled mild shocks of
short
duration and signaled shocks of longer duration and higher intensity,
the
animals still performed the response, thus apparently
subjecting
themselves to more punishment than was necessary. This type of vicious
circle behavior seems different from that of Brown et al.
Rather
than involve danger signals triggering SSDRs, it seems to
implicate
a tradeoff between severity of the shock and predicting when it ought
to
occur. On the other hand, Bolles also talks about safety
signals
that indicate a period free from danger. In the unsignaled condition, there
are no safety signals. Thus, this type of vicious circle behavior
may
well result from an organism's search for safety signals. That ought to
remind you a bit of the work on compensatory or antagonistic
conditioning, and its adaptive value: Being in a highly aroused and
tense physiological state because of the continual presence of danger
is
physiologically stressful; safety signals help moderate the wear and
tear.
Mediated Learning & Secondary Reinforcers
In classical conditioning,
we
discussed several types of mediated learning (higher-order
conditioning;
sensory preconditioning) involving building chains of associations
that would allow distant events to become associated together (recall
also
the Dwyer et al. study presented at the end of the last
chapter).
One of the major mechanisms for mediation in instrumental conditioning
is secondary reinforcement (although, as we will see, there are
certainly aspects of classical conditioning that govern this
mechanism).
Secondary reinforcement is learned reinforcement: an otherwise
neutral
stimulus that acquires the ability to motivate new learning or
performance.
Primary reinforcement, by way of contrast, is assumed to operate
reflexively because of an organism's genetic makeup.
Skinner may be
credited
with first making the distinction between primary and secondary
reinforcers.
The standard example of secondary reinforcers operating in human
societies
is the use of money. In our society, money includes round
pieces
of metal and rectangular pieces of paper that have an extraordinary
power
to motivate behavior. In other societies, different objects serve a
similar
function (tooled shells, for instance). These objects are not valuable
in themselves (aside from aesthetic considerations of design, etc.),
but
supposedly take on their value by means of serving as a medium of
exchange
for intrinsically valuable goods such as food or drink. Presumably,
they
acquire their reinforcing properties by being associated with primary
reinforcers.
Essentially, then,
secondary
reinforcers are believed to be conditioned through a process of
classical
conditioning involving the following set-up:
CS (neutral stimulus) & UCS (primary reinforcer such as food)
Once we have established a pairing between the CS and a primary
reinforcer,
we may then test for its value as a secondary reinforcer. Our
experimental
design would be as follows:
Group
Classical Conditioning
Instrumental Acquisition
experimental CS & primary
RF
R in presence of S+ followed by CS
control
(Nothing)
R in presence of S+ followed by CS
If we see an increase in responding in the experimental group
compared
to the control group, then our CS has acquired reinforcing properties.
This example should make clear why this is an instance of mediated
learning:
The effect of the CS in the experimental group occurs by virtue of its
link with the primary reinforcer or UCS. When that link weakens, the
value
of the CS as a secondary reinforcer ought also to weaken. Thus, in
times
of inflation when more money is required to buy the same food, the
reinforcing
properties of a dollar or five dollars weakens.
On a classical
conditioning
analysis of secondary reinforcement, we would expect to obtain findings
like those we've already seen in the previous chapters. Several
examples
of such findings might be mentioned. One involves a study by Egger
and
Miller. They trained pigeons using a design similar to this one:
Group Classical
Conditioning
Instrumental Acquisition
1
CS1 --> CS2 --> primary
RF
R followed by CS1 or CS2
2
CS1 --> CS2 --> primary
RF
R followed by CS1 or CS2
CS1 --> ..... --> no RF
As you can see from this design, Group 2 had discrimination training
in the sense that presence or absence of CS2 was
relevant
to predicting presence or absence of the UCS. Not surprisingly, given
its
better signal value, CS2 turned out to be the
secondary
reinforcer for the instrumental acquisition phase in this group. But
what
about Group 1? CS2 certainly has better contiguity
with
the UCS. However, in terms of signal value, it is not adding anything
to
what CS1 already predicts. Thus, it is redundant,
and
we would predict from models like Kamin's or Mackintosh's that CS2.
be blocked. Consistent with this prediction, the secondary reinforcer
for
instrumental acquisition in Group 1 is CS1, and not CS2.
Another example is rather
cute. It comes from the Brelands, former students of Skinner's
who
tried to train animals to perform in commercials using the principles
they
had learned. It is also cute because it involves the notion of money as
a secondary reinforcer. In one instance, they attempted to train a pig
to roll a (fake) coin into a piggy bank. During the training, the coin
was paired with a primary reinforcer, since they wanted to use the coin
as a secondary reinforcer for the responses involving in rolling. The
procedure
worked for a brief while, but then the pig started treating the coin as
if it were similar to the food it had been paired with: It tried to
root
the coin just as it would have rooted real food. This result, termed instinctive
drift, is perhaps one of the clearest demonstrations of the
involvement
of classical conditioning in secondary reinforcers, though it also
serves
to remind us that Watson's claim about reflexes quickly
becoming
overwhelmed by learned associations radically overstates the case.
Secondary reinforcers
play
an important role in certain aspects of therapy and classroom behavior.
In clinical and educational settings, behavior modification
techniques
based on principles of conditioning are used to try to change
unacceptable
behavior. These techniques typically include a component of secondary
reinforcement
by which objects such as poker chips may be accumulated for making
desired
responses (or avoiding undesired responses), and later traded for
privileges
such as snacks, movies, pencils, etc. Use of such secondary reinforcers
involves the construction of what is called a token economy.
Other findings relevant
to
the involvement of classical conditioning in secondary reinforcement
include
the intensity of the primary reinforcer (more intense primary
reinforcers
yield more effective secondary reinforcers), the number of times
the putative secondary reinforcer is paired with the primary
reinforcer,
and the delay between these events. You should be able to
figure
out why models such as Rescorla-Wagner or Wagner's rehearsal model, for
example, would support these findings.
One more important
phenomenon
while we are on the subject of mediated conditioning and secondary
reinforcement:
Most behavior involves a complex series of responses executed in a
certain
rapid and relatively smooth order. How is it that each
single
response can be reinforced? There hardly seems time for that. And how
is
it that organisms in real environments (rather than the
laboratory
where a researcher can control reinforcers and stimuli) acquire such
complex
organizations? The answer to these questions involves the concept of chaining,
and will prove to rely heavily on secondary reinforcers.
We briefly introduced the
notion of response chains earlier. An example will illustrate this
concept.
Let's set ourselves the task of teaching pigeons to Time Warp. The Time
Warp is the dance from the Rocky Horror Picture Show. It (as is
true of all dances) may be regarded as a series of steps in a chain. In
the case of the time warp, there are 5 steps (The Rocky Horror Show,
1975):
It's just a jump to the left, and then a step to the
right.
With your hands on your hips, you bring your knees in tight. But it's
the
pelvic thrust, They really drive you insane. Let's do the Time Warp
again.
Normally, we would try to teach a chain backwards, So, we will train
the
last step first. That involves teaching the pigeon a pelvic thrust. We
have our response here, but we need a stimulus and a reinforcer. Let's
use a red light for the stimulus (seems appropriate, huh?), and some
drink
for the reinforcer. Our design then is:
Phase Stimulus
(CS) Response
Reinforcer (UCS)
1
red
light
pelvic
thrust
drink
Note particularly that I
have also labeled the stimulus a CS, and the reinforcer a UCS. This is
meant to suggest that classical conditioning will be going on
simultaneously
with instrumental conditioning: The stimulus is paired not only
with
the response, but also with the outcome. Thus, as a result of instrumental
conditioning, the animal should do a pelvic thrust to the red light.
But,
as a result of classical conditioning, the red light ought to
become
a secondary reinforcer. And that should suggest to you the rest of the
design. Here it is in full:
Phase Stimulus
(CS) Response
Reinforcer (UCS)
1
red
light
pelvic
thrust
drink
2
blue
light
knees in
tight
red light
3
green
light
wings on
hips
blue light
4
yellow
light
step to
right
green light
5
white
light
jump to
left
yellow light
So, if you look for the moment just at Phase 2, notice that
we
will reward the pigeon for bringing its knees in tight by following
that
response with the red light. If the red light is a secondary
reinforcer,
then the animal will acquire the response. And note too that the red
light
also serves as the signal for the next step after knees in
tight:
the pelvic thrust. And finally, note that in Phase 2, we ought to
obtain
second-order conditioning: Two CSs (the blue and red
lights)
are being paired. If successful, this means that the blue light
now also becomes a secondary reinforcer.
At the end of this, the
sequence
will be that a white light serves as the signal for a jump to the left;
that's reinforced by the yellow light (thanks to fourth-order
conditioning)
which also signals Step 2 (a step to the right); that's reinforced by
the
green light (thanks to third-order conditioning), which signals Step 3
(wings on hips); that's reinforced by the blue light (thanks to
second-order
conditioning), which signals Step 4 (knees in tight); and that is
reinforced
by the red light (thanks to first-order conditioning), which finally
signals
the last step of the dance.
We haven't talked about a
control experiment for this, but our control would be something like
the
following:
Phase Stimulus
(CS) Response
Reinforcer (UCS)
1
red
light
pelvic
thrust
drink
2
blue
light
knees in
tight
green light
3
yellow
light
wings on
hips
white light
4
orange
light
step to
right
purple light
5
white
light
jump to
left
green light
In this control
experiment,
only the first step ought to be acquired. The secondary reinforcer from
the first phase is never used in the later phases, and none of these is
ever paired with a primary reinforcer. Indeed, based on work in
discrimination
training, we might predict that the other colors would become somewhat
inhibitory (since they tend to signal absence of UCS).
But in real-world chains,
of course, such individual discriminative cues and reinforcers do not
always appear to be present (although you could argue that they are
present
in a dance in terms of the auditory stimuli represented in the music!).
And we can solve that mystery by going back and analyzing responses as
having stimulus components. Responses are also being associated
with
a UCS, so that doing a response can act as a secondary reinforcer! Thus,
jumping to the left may be reinforced by stepping to the right,
eliminating
the need for all of these intervening light stimuli. If you thought our
pigeon caught in a very awkward situation, you were right: By
considering
the stimulus components of a response, we find a way to make the
concept
of response chains a lot more realistic, and their execution smoother.
Interference
Because of the nature of
instrumental
conditioning, it is possible to have several different responses
associated
with the same stimulus, or the same responses and stimuli associated
with
several different outcomes. Under those conditions, sometimes complex
patterns
of results may be found. In particular, certain combinations of events
appear to result in interference. We will consider two sorts of
interference
briefly in this section, and then revisit the issue in later chapters.
The two involve response competition and approach-avoidance
conflicts.
Response competition
involves
one response interfering with or competing with another. In fact,
response
competition is one of the theories regarding the process of extinction
(see the chapter on partial reinforcement and extinction). The
basic
idea here is that the animal is being cued to perform incompatible
responses.
An excellent example of
response
competition occurs in an experiment by Fowler and Miller. They
trained
rats to run to a goal box. During extinction, all of the rats were
shocked
on entering the goal box, but half of them were shocked on their front
paws, and the other half were shocked on their rear paws. The animals
shocked
on their front paws jerked back, whereas the animals shocked on their
rear
paws jerked forwards. Moving forward is a response compatible with
running
into the goal box, but moving backwards is an incompatible response.
Despite
the fact that both groups received shock or punishment for entering the
goal box, the front-paws group extinguished more rapidly. The new
response
caused by the shock in this case interfered with the old response.
Other examples of
response
competition come from work with humans in the verbal learning
paradigm.
Here, subjects are often asked to learn a list of word pairs,
and
tested on how successful they are at recalling the second word when
presented
with the first as a retrieval cue. So, if you studied a pair such as SHORT-LAKE,
the experimenter might say SHORT, and you would need to reply
with
LAKE. As you may gather, we can identify the first word of a
pair
as the stimulus term, and the second as the response
term.
Numerous studies show interference when we ask people to learn several
lists in which the same stimulus words are present, but there are
different
response words. Response competition will certainly not turn
out
to be the sole explanation of these findings (see, for example, Melton
& Irwin, and Postman's review). But it assuredly
handles
some of what is going on, as we find intrusions of the earlier
responses
during learning of the later responses.
As for approach-avoidance
conflicts, we may ask what happens when a response is associated
with
both an aversive and an appetitive outcome. That
situation
happens more frequently than you might think. In discrimination
training,
for example, we try to alter the excitatory generalization to the S-
by associating it with lack of a reward. But, that means that the
inhibition
building up for S- may also generalize to the S+,
canceling it out, to some extent (one of the explanations for peak
shift).
Thus, discrimination training involves two stimuli, each of which may
be
claimed to have some excitatory and some inhibitory components.
What ought to happen
should
thus reflect, in some sense, the summation of the excitation
and
inhibition, as was the case in use of the summation test in classical
conditioning
(see the discussion of algebraic summation theory in the
chapter
on attention and categorization for more details).
As an interesting
footnote,
Dollard and Miller tried to combine aspects of Freudian
psychoanalytic
theory and learning theory to describe some of the conflicts humans
might
be subject to. They identified several different types of conflicts,
but
one they termed an approach-avoidance conflict. In this
situation,
there is a tradeoff between the positive and negative components of
making
a response. As an example, we might take a rat running down an alleyway
to obtain some food. Suppose that the goal box is associated both with
food and with a shock. What will the rat do? One analysis of this
situation
(culled from several different studies) appears in Figure 2.
In this figure, we look
at
some measure of strength of a response as a function of how far from
the
goal the animal is. There are actually two opposed tendencies graphed
in
this figure: The tendency to approach the goal for a
reinforcer,
and the tendency to go away from the goal due to punishment.
The
solid line represents a typical, idealized avoidance gradient:
The
closer an animal is to an aversive or noxious stimulus, the more
vigorously
it leaves. As it gets further and further away, its response (running,
for example) gets weaker and weaker. In contrast, the approach
gradient
graphed by the dotted line demonstrates the reverse finding: the closer
to a desired reinforcement, the faster or more vigorously the animal
approaches
it.
Several additional
features
of Figure 2 are important. One is that the avoidance gradient is
typically
steeper than the gradient of approach, And the other is that in
this
figure, the lines cross. And because they do, we obtain an
approach-avoidance
conflict, with the spot at which the lines cross 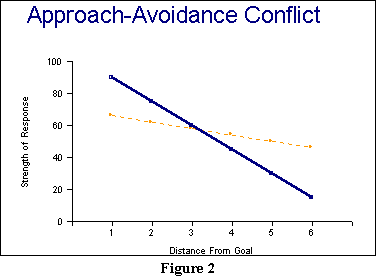 representing
the conflict point.
representing
the conflict point.
If you look to the right
of the conflict point, you will see that approach is stronger than
avoidance.
Thus, right of this spot, the animal should tend to head towards the
goal.
But once it passes the conflict point and approaches, then avoidance
becomes
stronger, driving it back. So, the model predicts that an animal will
waver
around the conflict point, developing large amounts of frustration in
the
process. There will be some tendency here for the animal to simply
escape
this situation, if that is at all an option.
Finally, an increase or
decrease
in the amount of reinforcement or punishment in this model will
essentially
move the relevant gradient up or down. Increasing the punisher, for
example,
should move the solid line up, and that will result in the conflict
point
(the spot at which the lines cross) moving further away from
the
goal. In like fashion, increasing reinforcement moves the approach
gradient
up, causing the spot at which the lines cross to occur closer to
the goal.
This is by no means all
that
Dollard and Miller have to say about what approach-avoidance conflicts
entail. You may be interested in reading their book on personality and
psychotherapy for more information.
The Partial Reinforcement Effect
A final basic phenomenon we
will discuss in this section involves the partial reinforcement
effect.
Skinner and his colleagues studied various aspects of
reinforcement-based
learning under what they claimed were 'real-world' conditions.
Specifically,
they asked what would happen to learning when reinforcers appeared on
only
some trials. The findings were quite interesting. Namely, with
partial reinforcements, there was greater resistance to extinction.
We will look at partial
reinforcement
in more detail in a later chapter. For now, let me mention that a
number
of variables interact with partial reinforcement. In particular, amount
of reinforcement will prove to play a pivotal role. In studies such as
those conducted by Roberts, we find that animals that have
been
continuously reinforced will display increased resistance to extinction
with small reinforcers. Roberts looked at extinction of alleyway
running
in rats whose reinforcers ranged from 1 to 25 food pellets. Over 36
extinction
trials, there was little evidence of a change in the 1-pellet group,
whereas
the 25-pellet group was performing at well less than half their rate
prior
to extinction. However, this effect appears to depend on how much
training
an animal has had during the acquisition phase; it assumes a fairly
substantial
amount of acquisition (see D'Amato). In contrast, animals
that
have been partially reinforced will display increased resistance with
large
reinforcers (see, for example, Ratliff and Ratliff). The
first
result, in particular, strikes many people as counterintuitive on first
coming across it. After all, shouldn't large reinforcers result in
better
learning, and shouldn't better learning be longer-lasting learning?
There are in fact a
number
of explanations for the partial reinforcement effect. For the moment,
however,
I will mention one to help you remember the results. This is Amsel's
Frustration Hypothesis, cited in the first chapter. According to
Amsel,
continuously reinforced animals will experience more frustration when
they
lose a large reinforcer. And since frustration acts as an aversive
stimulus,
these animals will avoid whatever it is that is causing the
frustration.
So, with more frustration, there is faster learning of avoidance. But
in
contrast, animals in partial reinforcement are being trained to
tolerate
frustration. With larger reinforcers, they are trained specifically to
handle greater and greater frustration. Thus, when they are placed in
the
highly frustrating situation of extinction (in which the expected
reward
fails to materialize), they will be better able to adapt to this
situation.
Number of trials during
acquisition
also has different effects for continuously and partially reinforced
groups.
For continuously reinforced groups, more training results in lesser
resistance, whereas for partially reinforced groups, more training
results
in greater resistance. The increased training in partially
reinforced
groups translates into better training to tolerate frustration, but the
increased training in continuously reinforced groups translates into
higher
expectation of a reward (and thus, a ruder awakening when it is no
longer
there).
In short, extinction is
frustrating,
because expected rewards don't occur. How much resistance to extinction
you will have will thus depend partly on how much frustration you
experience
during extinction, and on how much frustration you have been
trained
to tolerate during acquisition. The amount of frustration
experienced
during extinction depends on the size of the reinforcer you expected.
(Not
getting an expected $50 is a lot more frustrating than not getting an
expected
$1.) In addition, continuously reinforced animals have not been trained
to tolerate any frustration whatsoever.
C. Some Basic Paradigms
We have already introduced
two
general paradigms involving acquisition and extinction.
In
acquisition, an outcome is typically paired with making a response in
the
presence of a stimulus; in extinction, that pairing typically ceases.
Within
this broad framework (particularly with respect to acquisition), we may
distinguish several additional paradigms.
In appetitive or
approach learning, the animal makes a response that results in a
desired
reward. This is the type of learning involving reinforcement that we
have
implicitly and explicitly discussed so far. But it is not the only
paradigm
based on reinforcement. Another that deserves particular note is omission
training, in which an animal has to suppress or withhold a
response
in order to get its reward. Sheffield, for example, trained
dogs
to salivate in the presence of a tone associated with food, and then
shifted
them to omission training. In this latter phase, the dogs had to avoid
salivating to the tone for several seconds to get the food. Omission
training
is initially typically difficult, and displays a relatively slow
learning
curve. However, there are several studies suggesting that in the long
run,
it will be as effective as extinction in decreasing the frequency of a
response. Omission training is sometimes referred to as negative
punishment
to indicate that making the response is associated with removal of
a
reinforcer (which thus acts as a punishment).
Another paradigm based on
reinforcement is escape learning. In escape learning, the
animal
learns a response that gets it away from punishment, either by turning
off the punisher, or by allowing the animal to leave the area where the
punishment was administered. Escape learning is closely associated with
another paradigm, avoidance learning. In avoidance learning,
the
punishment is intermittent rather than continuous. If the
animal
makes the proper response before the punishment comes on, it
will
succeed in canceling that punishment. In avoidance learning, animals
typically
start out by escaping the aversive stimulation (making a response
during
the punishment that stops it), and then come to make the response early
enough that they subsequently successfully avoid the aversive
stimulation.
Punishment training
(or aversive learning), of course, involves the administration
of
an unpleasant, aversive outcome following a response. Thus, punishment
training, omission training, and extinction all have in
common
reducing the level of a given response, whereas appetitive
learning,
escape learning, and avoidance learning attempt to increase
response level. There are some obvious interplays in paradigms here,
depending
on which response you focus on. Often, aspects of several different
paradigms
combine: One response may be punished while another is reinforced.
We may also distinguish
between
signaled and unsignaled learning. A discrete, distinct
stimulus
is present in signaled learning, but not in unsignaled learning. Thus,
for example, in unsignaled avoidance, shocks can occur at
regular
intervals that could be avoided if the animal responds shortly
before
the shock's onset. There is no physical stimulus signaling the shock;
the
animal in this case needs to rely on an internal sense of time. In
unsignaled
conditions, features such as time or the contextual cues presumably act
as stimuli.
Another paradigm,
transfer
training will prove important, especially when we focus on
discrimination
in a later chapter. In transfer training, we look at the effects of
learning
one task on another. Transfer might be nonexistent (zero), positive
(facilitation: the learning is faster), or negative (inhibition:
there is interference). In addition, transfer effects might be proactive
(in which we look at the effect of an earlier task on the learning or
performance
of a later task), or retroactive (in which we saw how the later
task influences performance on the earlier one).
A final paradigm involves
shaping. Normally, approach learning applies to responses that
are
not especially frequent to start with, since we want to track an
increase in frequency as one of our measures of learning. Thus,
we find ourselves in the following situation: We sit in the lab,
watching
our animal subject, waiting for it to make the desired response so that
we can administer the reinforcer.
Such a procedure will
obviously
be inefficient. In some cases (such as a pig rolling a coin), the wait
may be very long indeed! Hence, a technology has developed that
involves
increasing the probability of having the animal emit that response so
that
we can then train it further through reinforcement. This technology,
called
shaping, requires reinforcing successive approximations to the
desired
response.
Shaping works as follows.
We start out by identifying a high-frequency component of the response
we want, and we reinforce that. So, if we want our rat to press a bar
on
the left side of an experimental chamber, then a high-frequency
component would involve having the rat be in the left half of
the
chamber. While it is exploring its environment, we reinforce for
crossing
over to the left. Then, as it increases its time on the left, we drop
the reinforcer. That will cause the behavior to become more variable.
We await some response yet closer to what we want to train (such as
being
near the bar), and when that occurs we reintroduce the
reinforcer.
And then, of course, we cycle the process through again in order to
obtain
yet a closer approximation (such as touching the bar). Shaping is a
very
powerful technique, not only because of its ability to 'coax' low
frequency
responses out of an animal, but also -- and especially -- because of
its ability to mold a response that is not normally part of the
animal's
repertoire! Thus, by combining shaping and chaining,
instrumental conditioning allows us to train totally new responses,
rather than just transfer stimulus control of an old response to a new
stimulus.
D. A Note About Terminology: Operant vs. Instrumental Learning
Finally, we ought to note a
distinction that is sometimes made between what is termed instrumental
conditioning, and operant conditioning. In instrumental
conditioning, the emphasis is on a discrete trial, a situation
in
which there is a clear starting point and a clear terminus.
We may measure how long it takes the animal to make the response during
the trial, or we may measure the relative probability of the animal's
success.
So, to take Thorndike's puzzlebox apparatus, the start of the trial
occurs
when a cat is placed in the puzzlebox, and it ends when the cat has
made
the escape response. How long this takes is what we are interested in.
Similarly, in maze learning, the trial starts with the animal being
placed
in the start box, and ends when the animal has found its way to the
goal
box. (Or alternatively, we can specify the trial as what happens in
some
amount of time from when we have placed the animal in the start box.
Where
has it gotten to in, say, an allowed 30 seconds? Learning here will
show
up as increased probability of having made the correct response within
the time frame of the trial.) In a third example, choice
discrimination,
the trial starts when the animal is exposed to two stimuli, and ends
when
the animal makes a response relevant to one of them. Our interest in
this
situation typically involves whether the animal has chosen the correct
stimulus.
There are no discrete
trials
in operant conditioning, on the other hand. A standard apparatus for
operant
conditioning involves a Skinner Box, a chamber with something
that
can be manipulated (a key to peck; a bar to press; a lever to move);
various
discriminative stimuli that may be turned on or off (lights; noises);
and
means to automatically administer reinforcements or punishments (food
or
shock dispensers connected to the bar, for example). Particularly with
respect to such simple responses as pressing a bar to obtain food, the
interest will be more in how rapidly those responses are executed. We
don't
stop the animal between responses in order to set up another trial.
Rather,
we typically look at characteristics of response rate over time.
This distinction between
discrete and continuous trials might also be expressed in a
slightly
different manner. On a discrete trial, you can succeed only once (or
perhaps
8 times if we use an apparatus like Olton's radial maze,
discussed
in the previous chapter), whereas on continuous trials you have the
opportunity
to obtain virtually unlimited reinforcements. So, the difference
between
instrumental and operant conditioning in part involves whether there is
a constraint on how many reinforcing events an animal can seek out.
That
having been said, I will generally treat these as equivalent.
II. Basic Requirements For Effective Conditioning
Many of the principles for
effective
conditioning will prove familiar from our discussion of classical
conditioning.
Thus, number of pairings of a response with an outcome will
prove
important in characterizing how quickly we see changes in
characteristics
of the response (its strength, its amplitude, its latency,
its probability, etc.) that signal evidence of learning. By the
same token, number of times a response fails to be followed by
an
outcome will be important, not only in describing the course of extinction,
but also (as in classical conditioning) in describing the contingency
between a response and an outcome. Below, we will briefly consider
additional
principles having to do with temporal contiguity, outcome
characteristics,
and contingency.
Before we do, however, we
ought to note several features that make the situation a bit more
interesting.
First, of course, is the issue of partial reinforcement. We
will
delay fuller discussion of that to a later chapter. Second, there is
the
fact that in operant conditioning, an animal is effectively in
charge
of whether to emit the response or withhold it. Obviously,
researchers
in classical conditioning may easily arrange pairings of the CS and the
UCS to achieve any desired contingency. But in operant conditioning,
controlling
how many times the reinforcer occurs when a response is emitted versus
when a response is not emitted is clearly trickier. Third, because of
the
presence of three events (stimulus, response, outcome), there
are three potential associations to worry about (S-R, S-O, and
R-O).
That means that we can ask about temporal contiguity (or contingency)
not
just of response and outcome, but also of stimulus and response, and of
stimulus and outcome. The situation thus becomes significantly more
complex.
Not all theorists believe
that all three associations form. Thorndike, to remind you,
accepted
only an S-R association, as did Watson. But, researchers such
as
Rescorla have made a very strong case that the other
associations
are there, as well. Thus, Colwill and Rescorla used the
devaluation
paradigm on a reinforcer after the response had been
acquired.
If a reinforcer's only function is to stamp in the association
(as
claimed by Thorndike), devaluing the reinforcer ought not to
influence
the response the animal gives to the stimulus. In the abstract, the
design
for this type of experiment would be similar to the following:
Group
Acquisition Phase Phase 2
Test Phase
experimental R to S for
RF
RF &
LiCl
R to S?
control
R to S for
RF
(Nothing)
R to S?
However, Colwill and Rescorla found a much less vigorous
response
following devaluation. This must have its effect on an R-O or S-O
association.
What about an S-O
association?
From our discussion of chaining and higher-order
conditioning,
you already know that this association forms. Further evidence of this
comes from the Rescorla study mentioned in the previous
chapter,
in which a stimulus that caused higher levels of responding during
extinction
became inhibitory, as measured by the summation and retardation tests.
We had earlier read about a classical conditioning version of that
study,
but Rescorla also ran the same study with an instrumental conditioning
set-up, and obtained the same results. Because the S-R association
in
these types of experiments is rapidly relearned following extinction
while
the S remains inhibitory, Rescorla claims that the inhibition doesn't
involve
the S-R link! And as a final example, consider a classic study by Seward
and Levy on a phenomenon termed latent extinction. In their
study, two groups of rats learned to run to a goal box for a reward.
Following
acquisition, one group had the experience of being placed directly in
the
goal without the reward. Then, both groups were put through extinction:
Group
Acquisition Phase
2
Phase 3
experimental run for
RF
put in goal, no RF
extinction
control
run for RF
(Nothing)
extinction
In this experiment, the control group extinguished more slowly than
the experimental group. Presumably, the stimulus elements of the goal
box
had now become associated with some inhibition for the experimental
group,
making their running to it less desirable.
Below, given the
theoretical
importance of reinforcement in operant conditioning, we will
concentrate
on principles having to do with its presence relative to the response.
A. Temporal Parameters
A principle of fairly long
standing
(and which forms a part of many behavior-level theories) has been that
there must be temporal contiguity between the response and the
outcome.
In fact, many studies report what is called a gradient of
reinforcement
in approach or appetitive learning: The longer the delay between the
response
and the reinforcer, the weaker the learning.
A well-known experiment
demonstrating
the gradient of reinforcement was conducted by Grice. Grice
used
a choice discrimination paradigm in which rats had to enter one
of two rooms or chambers. The rooms were different colors (black or
white),
and the rat was reinforced for entering one of these but not the other.
However, there were several groups of rats who differed in terms of how
long it took to get the reinforcer after choosing the correct color.
All
rats were immediately placed in a neutral-color room where the
reinforcer
was given, but one group received their reinforcer immediately, while
others
had to wait. The group with the longest wait was reinforced after 10
sec.
Essentially, Grice found a very rapid fall-off of learning. After about
1 sec, there was no evidence that the discrimination had been learned.
Depending on the response
and the circumstances (see the next major section below), longer delays
in which learning still occurs have been reported. As an example,
consider
a study by Capaldi, in which two groups of rats were trained to
run to a goal box. One group was rewarded as soon as it reached the
goal
box, but the other group had to wait 10 sec for its reward. Both indeed
learned to run to the goal box, but the running speed (and the initial
velocity out of the start box) was significantly depressed for the
10-sec
delay group. Thus, their learning seems to have been affected by the
delay.
Sometimes, a delay of 1
or
5 sec seems to result in no learning, and at other times (as in
Capaldi's
experiment), longer delays will be tolerated. Generally, however, the
speed
of learning as measured by vigor or probability of the response (or
number
of trials to acquire it) will be influenced by the response-reinforcer
delay. Extrapolating from Skinner's claims, we may present one
theory
for why this is so: Namely, as the delay period increases, the odds
increase
that the animal will perform some other piece of behavior before the
reinforcer
is given. The association may then form between that response
and
the outcome, rather than between the effective response and the
reward.
According to Skinner, temporal
contiguity by itself is all that is needed for the formation of an
association.
Skinner cites the example of superstitious behavior to
demonstrate
this. In superstitious behavior, animals are reinforced at random,
and need perform no response whatsoever. Yet, Skinner in one of
his studies reported that pigeons in this circumstance were displaying
apparently learned behaviors such as head shaking. He claimed that the
reinforcer by dumb luck must have been presented just after the pigeon
had tossed its head, so that head tossing was strengthened as a
response
in this situation. The increased possibility of acquiring superstitious
behavior that interferes with other learning might thus partly explain
why temporal contiguity is important.
From the perspective of a
more cognitive, representational-level approach, we may posit a
similar idea expressed in very different terms. Given the presence of a
reinforcer, the animal's task is to determine which of a number of
previous
responses might be the one that worked. As the number of responses
increases,
the task becomes more difficult. Moreover, because causes normally
result
in relatively immediate effects (excepting, of course, situations such
as illness or food poisoning: note the relevance to the taste
aversions
paradigm), organisms may be genetically predisposed to connect recent
behavior with the current outcome (a principle of causal
recency).
A similar principle, of
course,
applies to aversive situations. Fowler and Trapold in an
experiment
on escape learning varied how long it took for shock to turn
off
once the rat had run to a goal box. The best learning/performance
occurred
for a group of rats whose shock was turned off as soon as they entered
the goal box. Animals that had to wait a bit for shock to turn off did
worse.
Finally, Boe and
Church
found that the effectiveness of punishment decreased with delay. Unless
punishment is administered very shortly after an animal's response, it
will not prove very effective. Dog owners who come home and punish
puppies
for earlier 'accidents' are most likely to be associating themselves
with
the aversive outcome, and training fear of the owner and the spot where
the dog was punished. That is certainly not the same thing as
housebreaking
a pet.
B. Outcome Strength
Two types of outcomes have
generally
been discussed: reinforcement and punishment. Each,
however,
may be further subdivided into two sorts, positive and negative.
Positive outcomes generally involve the presentation of a stimulus that
changes a relatively neutral state into the state specified by the
outcome.
Thus, positive reinforcement (generally referred to without the
use of the word "positive") involves the provision of something
desirable
that normally results in appetitive behavior, and positive
punishment
(also typically referred to without the modifying adjective) involves
the
provision of something undesirable that normally results in aversive
behavior.
The other two types of
outcomes
are negative reinforcement and negative punishment. It
will
help you to keep these straight by recalling that anything that
is a labeled reinforcer, positive or negative, should operate
by
the law of reinforcement: It ought to increase the
response
that it follows. Similarly, anything labeled a punisher, positive or
negative,
ought to work by the law of punishment: It ought to decrease
the response that it follows. That having been said, a negative
reinforcer
takes on its reinforcing properties because some response the animal
makes
results in removal of aversive stimulation. Negative
reinforcement,
of course, is the basis for escape learning. And in similar
fashion,
a negative punisher acquires its punishing properties by virtue of the
fact that the animal makes a response leading to removal of a
reward
or privilege. Thus, positive outcomes involve the presentation
of stimulus events, and negative outcomes involve the removal
of certain stimulus events.
With respect to each,
there
appears to be a general principle that higher levels of strength result
in stronger or faster or more vigorous responding, consistent with a
claim
that outcome strength influences speed of learning. Concerning positive
reinforcement, for example, Kraeling taught three groups of
rats
to run an alleyway for a drink reinforcement that varied in the amount
of sucrose concentration (recall that rats have a sweet tooth, so
higher
sucrose concentrations act as more effective reinforcers). Each group
was
given one trial per day for 99 days. At the end, they had each reached
asymptote as measured by how fast they ran. However, the
asymptotes
differed for the three groups: The group with the highest
sucrose
concentration had the fastest asymptotic running speed whereas
the
group with the lowest concentration had the slowest
speed.
Crespi found similar results (see below, Figure 3): Rats given
large
amounts of reinforcement on each trial (64 pellets) showed faster
running
than rats given small amounts of reinforcement (4 pellets).
An experiment by Trapold
and Fowler can illustrate the operation of this principle with
amount
of negative reinforcement. They conducted an experiment in which rats
had
to run to escape shock. Five groups of animals were given 20 trials of
escape learning. The groups differed in the intensity of the shock
(varying
from 120 volts up to 400 volts). Faster acquisition of the escape
response
occurred with the larger shocks.
Finally, a classic
experiment
by Boe and Church may be used to illustrate the principle with
positive
punishment. Boe and Church trained four groups of rats to press a bar
for
a reward, and put each through extinction. Prior to extinction,
however,
three of these groups were put through punishment training in which,
for
15 minutes, a bar press gave the animal a shock. The groups differed in
intensity of the shock (35, 75, or 220 volts). Thus, the design was as
follows:
Group Acquisition
Phase
Punishment
Extinction Phase
1
RF for barpress (bp)
(None)
No RF for bp
2
RF for
barpress
bp --> 35 Volts No
RF for
bp
3
RF for
barpress
bp --> 75 Volts No
RF for
bp
4
RF for
barpress
bp --> 220 Volts No RF for bp
The question, of course, was how punishment of bar pressing would
help
speed up removal of that response. Over 9 sessions of extinction
training,
the group with the weak shock proved not all that different from the
group
with no punishment: Each engaged in a substantial number of responses
during
the course of extinction. However, quite different results occurred for
the 75 and 220 volt groups: They showed a much lower level of
responding
during extinction. Indeed, the 220 volt group hardly responded at all!
Thus, effectiveness of punishment in suppressing behavior will depend
in
part on severity of punishment. As the contrast between the control
group
and the 35 volt group demonstrates, weak punishers may have little
permanent
effect compared to extinction.
Of course, there are
other
variables that will influence the operation of an outcome. As you know
from an earlier discussion, aversive stimulation can have the
paradoxical
effect of increasing the response it is meant to stamp out (vicious
circle behavior). Also, the same amount of an outcome packaged in
different
ways may effectively act as different amounts. Thus, for example, Campbell,
Batsche, and Batsche found that a reinforcer divided into smaller
amounts
worked better than a reinforcer presented as one large amount. And to
remind
you, those manipulations that seem to promote higher asymptotic
levels during acquisition (in continuous reinforcement)
generally
also promote the fastest extinction.
There are also contrasts
that may occur when an organism experiences several different levels of
a reinforcement. An experiment by Crespi will illustrate these.
Crespi trained rats to run to a goal box for food (the
apparatus
here involved a straight alleyway in which rats are released at
one end of a corridor or tunnel, and have to run to the other end). One
group was given a large reward, a second group was given a medium
reward, and a third group was given a small reward. In each
case,
their running speed was measured. Then, the large-reward and
small-reward
groups were shifted to the medium reward. Thus, the design was
something
like the following:
Group Phase
1
(acquisition)
Phase 2 (maintenance)
1
64-pellet
reward
16-pellet reward
2
16-pellet
reward
16-pellet reward
3
4-pellet
reward
16-pellet reward
You will note that I have
labeled the two phases here acquisition and maintenance.
The rats in the acquisition phase received 20 learning trials,
and
their average running speed at the end of training was measured. In a maintenance
phase, on the other hand, we look at performance after learning has
occurred (that is, presumably after the association has formed). In
Crespi's study, there were 8 maintenance trials. Figure 3 presents the
results after learning, and on the eighth maintenance trial. As you can
see from this figure, Group 2 showed some slight increase; not
surprising,
since additional reinforced pairings ought to result in a stronger
association
according to most standard theories of instrumental conditioning. But
notice
what happened to the other two groups: A shift to a much smaller
reward caused a negative contrast by which running speed slowed
down considerably, whereas a shift to a much larger reward
resulted
in a corresponding increase (a positive contrast).
 It is important to note that all groups received the same amount of
learning in Phase 2 (in terms of number of trials and what the
reinforcer
was). Thus, we might have expected each group to display the same
relative improvement. But, that did not happen. Because these contrasts
occurred during a post-acquisition period involving identical
additional
training, they are generally interpreted as demonstrating an effect not
on learning (or acquisition), but rather on performance.
That argument is particularly compelling for Group 1: They continued to
receive additional reinforced training during Phase 2, yet they
apparently
got worse! Contrasts of this sort are termed incentive contrasts.
It is important to note that all groups received the same amount of
learning in Phase 2 (in terms of number of trials and what the
reinforcer
was). Thus, we might have expected each group to display the same
relative improvement. But, that did not happen. Because these contrasts
occurred during a post-acquisition period involving identical
additional
training, they are generally interpreted as demonstrating an effect not
on learning (or acquisition), but rather on performance.
That argument is particularly compelling for Group 1: They continued to
receive additional reinforced training during Phase 2, yet they
apparently
got worse! Contrasts of this sort are termed incentive contrasts.
Such contrasts should
suggest
that perhaps outcome amount is related more to an animal's motivation
to
perform a response than whether that response gets learned in the first
place.
Contrast effects may
occur
under a variety of conditions (see, for example, Flaherty's
review).
They do tend to be temporary, however. Flaherty suggests, in
particular,
that negative contrasts may reflect frustration at obtaining the less
desired
reward. Consistent with this, tranquilized animals generally do
not exhibit contrasts.
C. Contingency
We have already briefly
alluded
to the difficulties inherent in controlling contingency between a
response
and an outcome in operant conditioning. We have also briefly talked
about
the fact that Skinner (and Watson and Thorndike)
claimed
that contiguity was all that was needed for learning. As in classical
conditioning,
however, there are claims that contingency is also required for
learning
to occur. We will close out this section by considering the evidence
that
contingency influences learning and performance.
To start, let us adapt
the
notion of a contingency space discussed in the previous
chapter.
Previously, we had looked at the relative probabilities of the UCS when
the CS was present or absent. Now, we look at the relative probability
of an outcome when the animal makes a response (Probability 1),
or withholds it (i.e., does not make the response: Probability
2). Essentially, analogous to what happens in classical
conditioning,
many theorists will claim that when Probability 1 exceeds Probability
2,
there ought to be excitation: The animal is more likely to make
the response because the odds of getting a reward increase. (We are
assuming
rewards outcomes rather than punishers here!) In contrast, when
Probability
1 is below Probability 2, then it makes more sense for the animal not
to
respond: The response ought to be inhibited. Finally, when the
two
probabilities are equal (so that there is zero contingency), we would
expect
to find no evidence of acquisition.
How well does this notion
of contingency hold up? One interesting study that attempts to assess
this
notion of an operant contingency space was performed by Hammond.
Hammond set up different contingencies between bar pressing and a
reinforcer
for several groups of rats. Whenever the two probabilities were equal,
the rats failed to display any change in bar pressing. In these
circumstances,
there were always some pairings of the response with the outcome that
ought
to have resulted in the association forming, if Skinner's claims made
in
the section on superstitious behavior were correct. Indeed, we might
regard
such a circumstance as similar to a partial reinforcement schedule.
Nevertheless,
no evidence of learning occurred. In contrast, rats for whom
Probability
1 exceeded Probability 2 did increase their bar pressing.
Moreover, in a follow-up,
Hammond found that rats that had already acquired bar pressing stopped
when the two probabilities were made equal. This pattern of findings
certainly
goes against Skinner's claims that contiguity is sufficient. Instead,
it
strongly suggests an exquisite sensitivity to contingency on the rats'
part.
Why, then, do we obtain
superstitious
behavior? According to many people, Skinner has probably failed to
consider
the fact that reinforcers such as food also act as UCSs that elicit
certain
responses, and that the contextual cues act as a CS that gets
conditioned
to the UCS. On such an account, superstitious behavior really isn't: It
is fairly straight-forward classical conditioning of the sort we have
been
discussing in the last two chapters.
An example may serve to
drive
this point home. One claimed example of superstitious behavior has been
autoshaping. In autoshaping with pigeons some food is presented
after a key lights up (Brown &
Jenkins, 1968). After a while, pigeons peck the key, although
they
need not do so to obtain the food. Autoshaping is a very useful tool
for
training pigeons, because it seems that the pigeons will train
themselves,
and save you the work. But that avoids analysis of this situation as
classical
conditioning in which the lighted key serves as the CS. A nuanced
discussion of how classical conditioning can partly explain some of the
response characteristics (along with some of the problems for a simple
stimulus substitution view of classical condition) may be found in Staddon
and
Simmelhag (1971). Consistent with their discussion, Jenkins and Moore (1973), for
example, used either food pellets or drink as the reinforcer
for different groups of pigeons in an autoshaping paradigm.
Pigeons pecked at solid food with a closed beak, but opened
the
beak slightly to drink the liquid (you can see a demonstration of this
on YouTube by clicking here). Thus,
what appears to be contiguity without contingency
in operant conditioning is sometomes a classical conditioning situation
in
which there is both contiguity and contingency (but the
contingency
involves the CS and the UCS, rather than a response and an
outcome). On the other hand, Staddon and Simmelhag do point out
that such terminal responses
differ from the interim reposnses
that can be produced before them, and that do seem to better fit with
Skinner's notion of superstitious behavior.
Another experiment done
with
pigeons was performed by Killeen. The pigeons faced a
horizontal
array of 3 keys, the middle one of which was lit. They were trained to
peck at this middle key. About 5% of the time, one of their pecks at
the
key would cause its light to go off, and the lights of the two
surrounding
keys to come on. Another 5% of the time, a computer would automatically
turn off the center key while turning on the others. The question
Killeen
asked was whether the pigeon was aware that it was responsible for this
change.
How can we assess a
pigeon's
knowledge of the circumstances? Killeen reasoned that a pigeon aware of
whether its action had turned off the light would easily be able to
learn
another response that would depend on that action. So, the
experiment
was arranged so that the pigeon would get rewarded for pecking at one
of the surrounding lights when it was responsible for turning
them
on, but would have to peck at the other light when it was the
computer that had turned them on. That would be a difficult task to
accomplish without sensitivity to contingency, but Killeen's pigeons
came
through. They did indeed show they could discriminate events caused by
their own behavior from events caused by some other external cause.
Such sensitivity is not
reported
in all studies, however. In a quite clever study by Thomas,
rats
obtained random free reinforcements, but could also make a response
(bar
pressing) that would give them a reinforcement on demand. But the catch
was, the number of random reinforcers would drop some after the rat
made
that response. In other words, more reinforcers were available if
the
rat did not respond. In contrast to the studies above, the rats in
this experiment actually did learn to bar press, which resulted in
their
getting less food!
One more study on
reinforcement-based
contingency may be mentioned. This study, done by Watson (not
the
same Watson who redefined psychology as behaviorism!), used
3-months-old
human infants. Watson set up a contingency between their turning their
heads and a reinforcer of a mobile above their cribs turning for
several
seconds. A second group had the same experience of mobile reinforcer,
but
the second group's mobile movements had nothing to do with any of their
responses. Although both groups initially displayed a great deal of
interest
and pleasure in the mobile when it started moving, only the contingent
group maintained this reaction. Thus, Watson argued that the contingent
infants had some sense of mastery over the mobile that the
non-contingent
infants did not, some awareness that the mobile's movements were due to
their own actions. They were sensitive to contingency.
Contingency will also
prove
important in escape and avoidance learning. In particular, Seligman
and Maier have studied a phenomenon termed learned helplessness.
The experimental set-up for learned helplessness typically involves
something
like the following design:
Group
Phase 1
Phase 2
Experimental
inescapable,
noncontingent
P
escape learning
Control
(Nothing)
escape learning
They find that unavoidable, non-contingent punishment results in the
animals in the Experimental Group not learning to escape, once a
contingency
is set-up between an escape response and avoidance of the shock. The
Control
Group, in contrast, readily acquires the response. According to
Seligman's
explanation of these results (the cognitive deficit hypothesis),
the animals in the Experimental Group have acquired a mistaken belief.
Based on the randomness of the shocks and their inability to escape
them
in Phase 1, they have mistakenly learned that there is no response
that
will be effective in avoiding or escaping shock. (You may want to
compare
this to various explanations for learned irrelevance in
classical
conditioning.) Thus, they cease trying to discover an effective
response,
so that learning is no longer attempted in Phase 2. Seligman has argued
that some similar mechanism in humans may account for certain episodes
of depression.
III. Exceptions & Complex Interactions
We have seen, at least
implicitly,
an emphasis on reinforcement theories in which a reinforcer is
necessary
for appetitive or approach learning (see also Thorndike).
Indeed,
the notion of reinforcement is so pervasive that, as you will see in
the
next chapter, some theorists have even claimed that extinction depends
on there being a reinforcer present during the animal's extinction
training!
Although there are a variety of associational or behavior-level
theories
to account for instrumental and operant conditioning, we will direct
the
exceptions below to the most conservative of these: the claim that
learning
requires a reinforcer, needs only temporal contiguity, operates through
an automatic process of slowly strengthening an association, and stamps
in specific muscle movements that increase in likelihood in the
presence
of the S+.
A. Long-Delay Learning
We will start with the issue
of temporal contiguity, As was true in classical conditioning, we will
also find instances of long-delay learning in operant conditioning.
Indeed,
we might start by noting that the work of Garcia and Koelling,
presented
previously as an instance of long-delay classical conditioning, might just
as easily have been labeled instrumental conditioning: An animal
makes
a response of drinking saccharine -flavored water, and
experiences
an outcome of becoming ill several hours later. As this
reinterpretation
of the learned taste aversions paradigm illustrates, it may
sometimes
be difficult to draw sharp, clear boundaries between classical and
instrumental
conditioning (again, see Staddon & Simmelhag, 1971, for a
discussion of some of these issues; see also the last section in
this chapter).
Leaving aside the taste
aversions
work, however, there are other studies suggesting relatively long
delays
are possible. Lieberman, Davidson, and Thomas, for example,
presented
a series of experiments in which pigeons had to peck the right or left
side of a key. They found that some of their animal subjects were able
to learn the response even with delays of 7 sec or longer (an
extraordinarily
long delay for a pigeon). The animals that were able to learn were the
ones whose correct response was followed by an unusual event (a marker).
In their experiment, the marker involved the key briefly turning a
different
color (from white to red on its left half and green on its right half)
after it had been pecked. Other work by Lieberman and his
colleagues
has demonstrated that such marking can result in animals learning a
discrimination
even when the reinforcement is delayed a full minute. Since the marker
involved a non-reinforced stimulus occurring after the relevant
response but well before the reinforcement, the existence of a marking
effect poses a challenge to the idea that temporal contiguity is
always
necessary for learning.
Indeed, this study ought
to remind you a bit of some of the work we discussed regarding rehearsal
and surprisingness (in particular, the work by Wagner,
Rudy,
and Whitlow and that of Hall and Pearce). Surprising events
are apt to be rehearsed more. So, a distinctive surprising event
following
a response may result in that response being rehearsed for a longer
period
of time or becoming more distinct in memory (and thus more likely to be
sampled as the cause of the reinforcement). Lieberman et al.'s take on
this (the marking hypothesis) combines elements of both of the
above
(1985, p. 622):
[T]he effect of the marker proved to depend critically
on
what response preceded it: If a correct response was marked on food
trials,
then correct responding increased; if an incorrect response was marked,
then incorrect responding increased. The most plausible explanation
for this result, we believe, is that the marker triggered a memory
search
that focused attention on the preceding response, thereby increasing
the
likelihood that it would be remembered. [emphasis added]
Another example of
long-delay
learning concerns a study by D'Amato, Sarafin, and Salmon. They
delayed reinforcers by at least 30 minutes in training trials with
rats.
In one experiment, animals were placed in one of the two goal boxes of
a T-maze (an apparatus that looks like a T, in which the animal
runs from the start box at the base of the T to one of the two
arms
at the top), then put in the start box and fed 30 minutes later.
Despite
this delay, the animals exhibited differential running to the arm in
which
they had been placed. Note, in particular, that no additional events or
stimulus cues were present during the wait in the start box that may
have
become associated with the food: Once the animal was let out of the
start
box, the stimulus cues around the correct arm may have primed the
memory
of being in that arm.
Finally, note too that
the
work we have already mentioned by Olton using the 8-arm radial
maze
suggests that rats are quite adept at finding food in the maze without
retracing their steps, and without generally revisiting an
already-visited
arm. As they visit arms at random, they would appear to maintain some
information
in short-term memory concerning which responses have already been made.
Given the length of time it takes to visit all 8 arms, this clearly
qualifies
as a type of long-delay learning.
Numerous mechanisms for
long-delay
learning have been proposed. One that plays off of the notion of
secondary
reinforcers has been proposed by Spence. This involves the
mechanism
of an anticipatory fractional goal response (rg).
Note that the response in this instance is written with a lower-case r
rather than an upper-case R. The reason is that the r
is
treated as one component or fraction of a more complex
response,
the goal response (Rg), the animal makes on
reaching
the goal and obtaining its reward. There will be numerous fractions or
component responses such as chewing, swallowing, salivating, etc. These
get conditioned to the stimulus cues present shortly before the animal
enters the goal box. So, the association involves:
SGoalCues ----------> rg
But since these components or fractions are associated with food,
they
also become secondary reinforcers through higher-order conditioning.
Thus,
the cues present as the animal enters the goal area act as a reinforcer
before the animal has actually received any food on that trial.
In addition, these
components
also have stimulus properties they are associated with, although these,
of course are unlearned: Chewing, swallowing, etc., all cause certain
physical
sensations. So, the association ought also to include these, as follows
(the dots indicate an unlearned association):
SGoalCues ----------> rg ..... sg
And as was true of the response fraction, we indicate the stimulus
fraction
with a lower-case s. The rg ..... sg
is termed a mediator because it is a unit that may come between
a stimulus and a response in a chain of associations.
In Spence's theory, these
mediators become anticipatory; that is, they start being conditioned to
earlier and earlier spots in a sequence. Thus, in a complex maze, the
stimulus
cues right before the cues that led to the goal box also take on
secondary
reinforcing properties. If we regard the goal cues as being at spot X,
we will take the cues before these as being at spot X-1. Then,
through
classical conditioning we have:
SX-1 ----------> SGoalCues
---------->
rg ..... sg
Or, to represent this by a shortcut:
SX-1 ----------> rg ..... sg
And if at this point the animal needs to make a left turn to get to
the area of the goal box, then the associations at this point involve:
SX-1 ----------> rg ..... sg
----------> RLeft
And of course, we may now carry the procedure through to spot X-2
(the cues present before the X-1 cues). Thus, fractional
goal
responses are effectively conditioned throughout a complex chain in a
process
that should remind you of our example of the Time Warp. Consistent with
this theory, animals do tend to learn a complex maze backwards
(although
not all results support the theory: In particular, researchers have not
found evidence of anticipatory drooling at the various spots or choice
points of a maze).
Thus, in theory, the
presence
of secondary reinforcers may help to bridge what appears to be a long
delay.
That would mean that long delays are really much shorter, since we need
to assess the delay in terms of the first reinforcer present
after
a response. In this case, that first reinforcer will be a short-delay
secondary
reinforcer.
While secondary
reinforcers
and anticipatory fractional goal responses might account for some of
the
long-delay results, however, they cannot account for all of them. In
particular,
the study by D'Amato et al. would seem difficult to explain,
since
the animal is being fed in the start box, so that any secondary
reinforcers ought first to be associated with it, rather than
the
arm the animal runs to. Similarly, Olton's results would not
fall
under this mechanism, because secondary reinforcers ought to become
associated
with an arm the animal has already visited, making it more
likely
the animal will revisit the same arm on the next trial. But
that
generally doesn't happen. And that it doesn't happen makes sense,
according
to foraging theory: An animal foraging in the wild for food is
likely
to deplete a food source, so there is adaptive value in searching for
food
in different locations. But, searching for food in this manner also
requires
a memory system that can keep track of where food was found previously,
so as to avoid that spot.
Other factors that make
long-delay
learning possible include the presence of something to make the correct
response distinct, or the occurrence of little intervening
activity
between the effective response and the reward. We have already
discussed
distinctiveness in terms of Lieberman's marking hypothesis:
A distinct response is likely to be more salient, rehearsed more, and
thus
more readily available in memory when the occurrence of a reward
triggers
a search for events that might have been responsible for it. The notion
of little intervening activity will similarly play off of a memory
mechanism.
With little or no intervening activity, the last response will still be
the one most likely to be recovered from memory. But as activity
increases,
so do the possibilities of disruption (recall what Wagner, Rudy,
and
Whitlow found with post-trial episodes!), and choosing the
wrong
response as being the cause of the reward (response competition).
B. Belongingness
On a strict associational
account,
any association ought to form between responses and effective
outcomes.
However, several studies (in addition to the work we have already
discussed
in learned taste aversions) suggest that need not be the case.
One of these studies,
conducted
by Shettleworth, involved an experiment with hamsters. Shettleworth
identified six high frequency activities in hamsters that included face
washing, digging, scent marking, hind leg scratching, rearing, and
front
paw scraping. When each of these was subsequently paired with a food
reinforcer,
only digging, rearing, and front paw scraping were affected. Such
restriction
of the operation of a reinforcer represents a violation of the
requirement
that reinforcers be transituational: Here, at least, are three
responses
that a reinforcer of food will not affect.
Another study
illustrating
belongingness comes out of the work of Premack. By allowing
kids
to play with gumball machines (dispensing candy) and pinball (game)
machines,
Premack identified kids who were players or eaters (based on the
relative
proportion of time they spent with each machine). He then set up an
experiment
using the following design:
Group Subjects
Response & Reinforcer
1A
players
play to eat
1B
players
eat to play
2A
eaters
play to eat
2B
eaters
eat to play
Thus, there was now a contingency between responding on one machine,
and responding on the other: In one case (play to eat), kids
would
have to increase their time on the pinball machine to get an
opportunity
to use the gumball machine; in the other (eat to play), the
reverse
was required: kids would have to increase responding to the gumball
machine
to get a shot at the pinball machine. Only groups 1B and 2A showed
learning.
Thus, what counts as an effective reinforcer for one child may
be
completely ineffective for another. (Similar results hold up
for
animals: see the next chapter).
Also as a potential
illustration
of belongingness, we might mention the fact that certain
responses
that are easy to acquire with positive reinforcement become very
difficult
to acquire with negative reinforcement. Pecking a key for
pigeons,
for example, is difficult to train with negative reinforcement (e.g., MacPhail).
The explanation for this latter result may have to do with Bolles's
theory of safety and danger signals. Negative reinforcement
involves
the presence of danger signals that trigger SSDRs. Such
responses
may well interfere with the desired response, particularly
if that desired response involves approaching the danger signal
or aversive stimulus! Such a notion is similar to a more general principle
of preparedness posited by Seligman: Responses may be
ordered
on a continuum ranging from prepared responses at one extreme
to
contraprepared responses at the other. Prepared responses are
responses
quite similar to what an animal would naturally do in a given
situation,
whereas contraprepared responses are those the exact opposite of what
the
animal would normally do (approach danger rather than flee from it, for
example). According to Seligman's principle, the closer a response is
to
the prepared end of the continuum, the easier it should be
learned.
So, the exact same response may be acceptable in one circumstance, but
not in another.
C. Acquisition Without Direct Reinforcement
A number of studies question
whether a reinforcer is necessary for forming or strengthening an
association.
The classic experiment illustrating this phenomenon was done by Tolman
and Honzik. Their design involved having rats learn a maze. The
rats
were given one trial per day for 17 days. The experiment involved the
following
design:
Group
Treatment
1
no RF; removed when reach goal box
2
RF on each day when reach goal box
3
no RF until the 11th day
The question Tolman and Honzik asked was how the third group
would perform on days 11 through 17. Since these days represented the
first
time this group had experienced reinforcement, a reinforcement-based
account of learning would suggest that these animals started
learning
only on Day 11. But in fact, on the 12th day, these animals
were performing as well as (in fact, slightly better than) the animals
reinforced from the beginning (Group 2): They had learned to navigate
the
maze in the absence of a reinforcer. This finding, termed latent
learning, suggests that reinforcement may be more important for performance
(motivating an animal to show its knowledge) rather than acquisition.
Another similar result
involves
a study by Butler in which monkeys learned a response whose
consequence
involved being given access to a window looking out on a parking lot.
While
curiosity might be called a reinforcer, it seems a bit of a
stretch
in this case. The problem is that we have no way of independently
identifying
when learning would be expected to occur in the absence of any other
reinforcer
such as food, and when it would not. When is the animal curious?
A third study involves
the
area of observational learning. In a famous experiment by Bandura,
kids watched a tape of a clown playing with toys. Children in the vicarious
reinforcement group saw the clown being rewarded, but children in
the
vicarious punishment group saw the clown being punished for the
way he played. Later, when these kids were given a chance to play with
the same toys, the kids in the vicarious reinforcement group displayed
the same behaviors: evidence that they had learned by watching.
The kids in the punishment group played in a very different manner. But
they had acquired the responses as well: When the experimenter asked
them
to show what the clown had done, they were able to do so. Thus, we find
from this study that reinforcement and punishment may have an effect at
a distance: Watching others be reinforced can serve as a reinforcement.
Such a notion takes us far afield from the original idea of an
appetitive
stimulus that follows an emitted response (note that the children had not
made the response themselves, and note also that all children
had
learned the response, though some of them had suppressed it until given
permission by the experimenter to play the way the clown had played).
A final study on
observational
learning illustrates that the notion is not restricted to humans. Kohn
and Dennis taught one group of rats a choice discrimination.
A second group that were able to watch the training of the first group
learned the choice discrimination faster. Both the Kohn and Dennis
and the Bandura studies suggest a point we will explore in the
next
subsection; namely, that responses do not need to first be emitted in
order
for learning to occur.
D. Non-Response-Dependent Acquisition
There are several
embarrassments
for a theory that claims learning requires making a response. One such
embarrassment is perhaps more severe than another, but we can
categorize
these into two sorts: learning prior to responding, and
response
variations during acquisition or performance.
Learning Prior To Responding
As we saw in the
observational
learning studies, organisms (human and non-human) can start the process
of learning prior to actually performing a physical response. To
reiterate
a theme that was important in classical conditioning, this seems
strongly
to suggest that an association forms between mental representations.
Let's consider three more examples of this. They are relevant because,
unlike the Bandura and Kohn and Dennis studies, they
will
not involve learning by observing others perform (i.e., learning
by imitation). Thus, they cannot easily be accounted for in terms
of
vicarious reinforcement.
The first is a study by McNamara,
Long, and Wike. They looked at how long it took to train two groups
of rats to learn a maze. One group, however, was initially placed
one-by-one
in a 'wagon' and dragged through the maze. This group did not perform
any
of the running or turning responses, but they did get to observe their
environments. And as you have probably guessed, this group learned to
run
to the goal box (where they had been dragged) faster than the group
without
that experience. Presumably, while being dragged through the maze, they
had opportunities to observe the various stimulus cues and form a
representation
of their environments (a cognitive map: see the discussion
below
and in the next chapter on Tolman's theory of learning).
Learning
to navigate through those environments was thus speeded up for this
group.
The second study involved
the acquisition of cognitive maps by chimpanzees. In this study, Menzel
had animal subjects watch while food was hidden in slightly under 20
different
locations in a large field. The animals were brought along as Menzel
hid
the food over the field. Subsequently, they were released at the
starting
point, and observed while they collected the food. Two findings are
relevant
here. First, they knew the locations of the food, despite having
made
no response themselves. And second, they did not collect the
food
in the same order as it was hidden. Thus, we would not want to
claim
that a path through the field (consisting of a chain of locations to
visit)
was learned (as may have been the case in the McNamara et al.
study).
They clearly did not imitate Menzel's path or chain in this instance.
The
results again seem to suggest that animals can acquire representations
that are map-like, and that their learning will show up as an enhanced
ability to successfully find food (rather than as the performance of a
given order of responses).
Our third study was
introduced
earlier in this chapter. This is the study on latent extinction
by Seward and Levy. To remind you, animals placed directly in a
goal box in which they have previously been fed more rapidly
extinguished
running to that goal box than a group without this initial experience.
Insofar as extinction is normally defined as requiring
the
process of making a non-reinforced response, both groups
should have extinguished at the same rate: The initial
experience
of the experimental group did not involve making a non-reinforced
running
response! But that didn't happen. Presumably, through classical
conditioning,
the goal box had become associated with food, so that absence of food
may
have aroused frustration or inhibition. Thus, a change in the value of
the outcome (a type of devaluation) altered its value for the
experimental
group, making learning of extinction easier.
Response Variations
Alternatively, we may ask
whether
a given response, once it is acquired, is stamped in. On a strict
associationist
account such as Watson's or Hull's, motor movements are trained.
However,
the evidence strongly seems to disconfirm this, too.
Consider a study by Macfarlane.
In this study, rats were trained to run a T-maze. Once they had
acquired
this response, Macfarlane flooded the maze and put the rats into the
start
box. They swam to the goal box that had previously been
reinforced.
The point of this study, of course, is that swimming and running
technically
involve different muscle movements. So, if Watson's view of
learning
were correct, we ought not to find evidence of learning when a
different
response is executed. But consistent with our discussion of cognitive
maps, these animals had learned where to go: How to get there was
not
all that important.
A quite similar point
occurs
in studies with the use of the Morris maze. The Morris maze is
a
pool of opaque, milky-white liquid that has a platform somewhere
underneath
the water. The platform is close enough to the surface to enable a rat
to keep its head above water without having to swim. Morris and
his colleagues have found that rats released into the pool from the
same
spot eventually discover the platform (not having to expend energy on
swimming
is the reinforcer), and then learn to swim straight towards it. What is
important from our perspective, however, is that these animals still
head towards the platform when they are released from a new location:
They are able to adjust their angle of swimming relative to the
landmarks
in the room that tell them in what direction the platform ought to be.
That technically involves a different response then the one
these
animals made during acquisition. That they can execute the proper novel
response again illustrates the involvement of cognitive maps in
learning.
What drives performance here is where to get to, and how to get there
as
soon as possible.
And indeed, people who
study
acquisition often report that animals will perform a number of
different
physical responses that appear equally effective in obtaining
reinforcement.
Rats need not (and will not) always press the bar with the same paw.
Finally, although it
takes
us slightly off of the focus of this section, we may also mention
studies
that show animals do not always prefer to make a response that has just
been reinforced (and that should therefore be relatively strong). On a
T-maze, for example, rats have a tendency to visit alternate arms
(e.g.,
Dember and Fowler). This should remind you of our discussion of
foraging theory. Similarly, Harlow has demonstrated that
primates can learn a win-shift lose-stay strategy in choice
discrimination
in which they have to select the non-reinforced stimulus on the
next trial. Responding to the previous S- and avoiding a
response
to the previous S+ ought to be difficult under normal associationist
assumptions.
Under foraging theory assumptions, it ought not to be that difficult.
E. Multiple Stimuli & 'Compound Conditioning'
Stimuli are complex events
that
can be broken down into yet simpler stimuli. Moreover, several
different
stimuli may be present on a given trial, each associated with its own
reinforcer.
In this section, we consider what happens in these circumstances.
Herrnstein's Matching Law
Consider the following
situation:
A pigeon is trained to peck at a key for reinforcement. We use a variable
interval schedule (see the chapter on extinction and partial
reinforcement for more details) in which the first peck after some
random interval of time is reinforced. There may be a red key in one
block
of trials, and the first effective peck at it will yield two
reinforcers.
In another block of acquisition trials, there may be a blue key that
will
be rewarded with one reinforcer when pecked. And finally, in yet
another
block of trials, a green key may be associated with five reinforcers.
Following
this training, what will happen when the pigeon is put into an operant
chamber containing all three keys? Situations such as this were
investigated by Herrnstein.
The answer may surprise
you.
(And it will perhaps startle you to find that the answer doesn't depend
on species: College-level humans have displayed the same result!) A
first-guess
common-sense theory most people come up with is that the animal (or
human)
will spend all of its time on the key that has the best value -- the
green
key. But this often does not happen. Instead, the animal (and
the
human) will distribute its responses in proportion to the
reinforcements
available. That is, it will peck all of the keys over a period
of
time, but will peck the green key proportionately more often
than
the red key, and the red proportionately more often than the blue.
Herrnstein has called
this
the matching law. To provide a simple formula for this law, let
us assume we have a series of possible responses corresponding to a
series
of stimuli. In that case,
Responses to S1/Total Responses = RFs from S1/Total
Available RFs
So, to calculate the proportion of times our animal spends with each
key in the example above, we would calculate the following:
Proportion of Responses to SGreen = 5/(5+2+1) = 5/8 = .625
Proportion of Responses to SRed = 2/(5+2+1) = 2/8 = .25
Proportion of Responses to SBlue = 1/(5+2+1) = 1/8 = .125
That is, it should distribute 62.5% of its responses to the green
key,
25% to the red key, and only 12.5% to the blue key.
The matching law applies
generally whenever there is a difference in value of the reinforcer. We
know that temporal contiguity can affect the value of a reinforcer, and
a version of the matching law has been formulated for this situation,
as
well. But in this case, value depends on the reciprocal of the delay.
A reinforcer that is given after a short delay has more value
than
one given after a long delay. So, given the same reinforcer
presented
at 2 and at 8 sec delays, its value would be 1/2 (.5) and 1/8 (.125),
respectively.
In this case, the matching law would have the following formula:
Responses to S1/Total Responses = value of RF from S1/Total
Available values
Let us take another
example.
We'll again use a pigeon trained to peck at a red, blue, or green key.
This time, the pigeon gets the same reinforcer from each, but
at
different delays: 2 sec for pecking at the red key, 4 sec for
pecking
at the blue key, and 8 sec for pecking at the green key. Before even
doing
any of the calculations, you ought to correctly predict that the red
key
will be pecked most (fastest reward), and the green key least (slowest
reward).
But let's do the
calculations.
First, we need to calculate the values based on the delays. Remember
that
these are reciprocals. So, the values are:
red: 1/2 = .5 blue: 1/4 = .25 green: 1/8 = .125
Plugging these into our formula will yield the following results:
Proportion of Responses to SGreen = .125/(.125+.25+.5) =
.125/.875
= .143
Proportion of Responses to SRed = .5/(.125+.25+.5) = .5/.875
= .571
Proportion of Responses to SBlue = .25/(.125+.25+.5) =
.25/.875
= .286
You can see that the our
predictions turn out to be correct. Specifically, our pigeon ought to
distribute
57.1% of its responses to the red key, but 14.3% to green. (As a check
on your calculations, by the way, note that the values ought all to add
up to 100%!)
The matching law also
applies
to aversive outcomes. However, there is some evidence that it does not
apply to all instrumental situations (see, for example, Allison).
Some discrepancies have been found with fixed interval schedules,
for example (see the chapter on extinction and partial
reinforcement).
Nevertheless, it illustrates complex processes that depend on comparing
momentary values from multiple stimuli. Several theories have been
proposed
to explain the matching law. One of these, the melioration theory,
claims that animals are assessing the momentary odds of a payoff. When
one stimulus has paid off, then the animal works on the stimulus that
is
next most likely to pay off. Although the example differs somewhat, it
is reminiscent of people playing multiple slot machines who shift to a
different machine as soon as the machine they're on has scored.
Compound Conditioning
The phrase compound
conditioning
is typically discussed in reference to classical conditioning.
Nevertheless,
it is also appropriate here, as there are many instances in which
several
stimulus elements are present during learning. The typical (and not
always
correct) assumption is that a complex stimulus is the basis for generalization:
The animal has associated a response with all of the elements
of
that stimulus, and generalizes its responses to other stimulus
complexes
as a function of how many elements overlap. However, we sometimes
obtain
results reminiscent of blocking or overshadowing. We will look at these
in more detail in the chapter on attention and categorization,
but let us briefly consider several studies that will illustrate the
point.
The first is a study by Reynolds.
Reynolds taught pigeons to peck at a key that included several
different
stimulus elements. Specifically, there was a white triangle against a
red
background on top of the reinforced key (as opposed to a circle against
a green background for the non-reinforced key). In a later
generalization
test, Reynolds checked for how many pecks the pigeons would give to a
completely
red key, a completely green key, a triangular key, and a circular key.
If the pigeons were under the control of the total stimulus complex
(triangle
& red), then we would expect significant generalization to both red
and triangle, since they contain elements of the original training
complex.
Instead, she found that one pigeon pecked just to the red key, and the
other pecked just to the triangular key. In this case, one element of
the
complex was the only effective or salient element; it completely
overshadowed
the other.
A second study that
illustrates
a similar phenomenon was conducted by Wagner, Logan, Haberlandt,
and
Price. They set up a design for two groups of animals that was
something
like the following:
Group Compound Stimulus
RF Frequency
1
Light & Tone1
50%
Light & Tone2
50%
2
Light & Tone1
100%
Light & Tone2
0%
This study, of course, manipulates signal value. Thus, for
the
first group, the light has better signal value than either of
the
tones, because the light predicts more reinforcers (each tone by itself
only predicts half the reinforcers the light does; you would
have
to attend to both tones to predict as many reinforcers as the
light:
two things to track rather than one). In contrast, the light is
a worse predictor than Tone1 in the second group:
paying
attention to the light will work only half the time (as it does for
Group
1), but paying attention to Tone1 will work all
of the time. Wagner et al. find that the light does a more
thorough
job of controlling responding in the first group, despite the fact that
it really predicts the same number of reinforcers in both (note the
similarity
to how we set up a blocking design in classical conditioning).
Can we get blocking in
the
traditional fashion? We ought to predict a blocking effect if
instrumental
conditioning operates the same way classical conditioning does. That
is,
given the following design:
Group Phase 1
Phase 2
1
RF for R to S1
RF for R to S1&S2
2
(Nothing)
RF for R to S1&S2
we would predict that the animals in Group 1 should block to S2,
whereas the animals in Group 2 might be expected to show some
conditioning
to both S1 and S2. Thomas,
Mariner,
and Sherry used this type of design with pigeons, and obtained
evidence
of blocking.
Finally, consider a study
by Lawrence and DeRivera. They used stimuli involving different
shades of grey. In this study, animals had to perform one response if
the
darker shade was on top of a lighter shade, and the opposite response
if
the lighter shade was on top of the darker. During training, the bottom
shade in all cases was a neutral grey.
The question Lawrence and
DeRivera asked was whether the animals were under control of just
the top color, or were comparing the top color with the bottom
color.
To make a long story short, they found that animals were essentially
comparing
the top shade to the bottom shade. When two of the lighter shades were
presented, for example, which response the animal performed depended on
whether the lighter of these shades was on the top or the bottom, even
though both shades had been associated with the same response during
acquisition.
In this case, responding was apparently based on a comparison rule such
as darker on top or lighter on top. Such comparative
sensitivity
is referred to as relational learning. It is incompatible with
the
claim that an association forms between independent stimulus elements
and
the response. That assumption forms part of many associationist models
such as Hull's or Spence's theories (see the next chapter), but it also
appears inconsistent with a model like the Rescorla-Wagner model,
in which each stimulus is treated independently of the others on a
conditioning
trial. Under certain circumstances, stimulus configurations are not
equal to the sum of their component parts.
IV. Several Views Of Instrumental Conditioning
We have implicitly and
explicitly discussed several views of what instrumental conditioning
involves.
On many associationist accounts, there will be an association between a
stimulus and a response, at a minimum, and there may or may not be
associations
with the outcome. Learning involves the formation of these
associations,
or their strengthening or weakening. In contrast, as many of the
studies
we have just reviewed on cognitive maps and response variations
suggest,
the stimulus-response link may not be all that critical. In this
section,
we briefly introduce two diametrically opposed views of learning. These
are the radical behaviorist approach of Skinner and the
cognitive
expectancy theory of Tolman.
A. Skinner's View
We start with a notion
Skinner
expresses that makes him fundamentally different from
practically
every one else in the field of learning: Skinner's refusal to engage in
formal theorizing. For Skinner, theories involve hypothetical
constructs
that cannot be directly observed, and that therefore ought not to be
discussed.
And since theories (for most scientists and researchers) generate
predictions
to be tested, you may correctly guess that Skinner's notion of what
experiments
are about will also differ. Perhaps the best way of putting it is this:
Skinner's system only requires that we be able to predict and
control
behavior. When we have accomplished that goal, we need go no
further.
So, we do experiments to see which environmental contingencies control
behavior in which ways.
For Skinner, there are
essentially
two broad categories of behavior: operants and respondents.
Respondents cover behaviors that are reflexively coaxed out of the
animal
by the presence of specific stimuli. The presence of other
stimuli
at the same time enables them to acquire the ability to elicit
these
responses. Thus, he includes the work in classical conditioning
under the category respondent. But operants are more
characteristic
of what most of us (and most higher animals) do: They are the pieces of
behavior that an animal emits in a given situation.
The distinction between elicited
and emitted behavior is an important one for Skinner. Emitted
behavior
is not triggered by a single stimulus link to that behavior, whatever
it
is. Operants are presumably influenced by a whole complex of events
including the contextual stimuli, genetic constraints, and motivational
factors having to do with the animal's state of hunger or thirst at the
time. This is such a complex set of determiners that for all practical
purposes, Skinner refuses to talk about which stimuli connect with
which
responses. Thus, contrary to most behaviorist theorists, Skinner
does not talk about an association forming between a given stimulus and
a given response, nor about whether and when that association strengthens
or weakens. Rather, stimuli for Skinner serve something of the
same
function as occasion setters: They signal times at which a response-outcome
contingency occurs.
We ought to take a moment
to note that the notion of a response-outcome contingency in Skinner's
work does not mean the same thing as an operant contingency
space.
This notion merely means that the experimenter has set up a condition
whereby
a given response will occasionally be followed by an outcome. Contiguity
of the response and the outcome constitute the relevant learning
mechanism,
as we have seen in the previous discussion of superstitious behavior.
But that is not to say that stimuli are irrelevant. When an outcome
follows
a response in the presence of one stimulus but not another, the animal
learns a discrimination. The response comes under the control of the
first
stimulus (stimulus control), not in the sense that there is a
triggering
effect, but in the sense that the first stimulus becomes part of the
entire
stimulus situation in which responding alters.
We ought also to take out
a moment to discuss this notion of a response. Although Skinner
did use the term, it is in some sense odd, given the de-emphasis on any
specific cause of the response. Given his view, it will not surprise
you
to learn that he did not insist that learning require the same physical
response to increase or decrease in frequency (unlike Watson
and
Hull, who both claimed an association formed with specific
muscular
movements). So, many of the objections we looked at above to the
learning
of specific movements do not apply to Skinner. Instead, he
adopted
a functional definition of a response: any set of responses
that
achieve the same function qualify as the same response. Thus, if
the
function is to get to the left side of a T-maze, running to it,
swimming
to it, backing up to it, and casually crawfishing to it all count as
the
same response, because they all accomplish the same function.
Moreover, through shaping
and chaining, complex operants followed by a single reinforcer
are
strengthened as a group, so that behavior may be constituted into quite
long sequences. The sequence of getting out your car key, inserting it
into the lock, unlocking the door, taking the key out, opening the
door,
getting in, closing the door, putting the key in the ignition, and
turning
it may all be reinforced by the motor coming on, but will all be
punished
by an engine that refuses to turn. If this seems a strange example to
bring
up, it isn't, really. Above all, Skinner was always concerned with the
practical aspects of modifying behavior in the real world, and
exploring
how real-world contingencies affected behavior. Thus, in part as a pragmatist,
he was concerned with what worked, and not with elaborate theories of
why
or how.
Perhaps because he was a
pragmatist, he and his followers also tended to avoid experimental
designs
involving large groups of animals or people. His focus was on the
individual,
and whatever changes could be observed in the individual. Control that
individual's behavior to some extent, and you have demonstrated
sufficient
explanation for why it occurs (since you are now able reliably to
predict
the presence or absence of that behavior).
Among his contributions
was
the study of partial reinforcement schedules (see Ferster and
Skinner),
behavior modification techniques and token economies,
the
notion of superstitious behavior, behavioral-level
definitions
of reinforcers and punishers (as opposed to the
theoretical
definitions we will see in the next chapter), work on secondary
reinforcers
and punishers, teaching machines, and the notion of negative
reinforcement (which involves a different definition than the one I
have used earlier: For Skinner, negative reinforcers are aversive
events
that operate as reinforcers by being removed. This is a subtle
difference,
but recall that we have defined negative reinforcement in terms of the
removal of the event, not whether the event itself is aversive).
Skinner, unlike Watson, was also happy talking about the conditioning
of
private events. And shortly before his death in 1990, he
lambasted
the current emphasis in American Psychology on cognitive models and
memory
systems. Ironically, his approach had become isolated from mainstream
research
at the same time that his behavior modification procedures had become a
normal part of educational and clinical management techniques.
I don't think Skinner
would
have been disturbed by any research finding whatsoever. Since he
refused
to build formal theories, no finding could really have been
inconsistent
with his approach. In some sense, I think of Skinner as engaged in a
process
of cataloging or categorizing behavior: What are the situations under
which
this response increases? What are the situations under which it
displays
resistance to extinction? What mechanisms are effective for altering a
response? How can we set our environments up to provide maximal
efficiency?
These were the issues that occupied him.
As an example of work
inspired
by Skinner's approach, we may consider a famous series of experiments
by
people like Verplanck and Greenspoon on verbal
conditioning.
They used a reinforcer of agreement (e.g., "uh huh" or even a
pencil
tap) and showed that people increased whatever it was that the "uh huh"
followed: plural nouns rather than singular nouns, affective rather
than
descriptive statements, etc. Following acquisition, Greenspan put his
subjects
through extinction, and following that, questioned them to see if they
had been aware of what was going on. He claimed they weren't. Thus,
several
theorists made the very strong claim that humans could easily be
conditioned
without their awareness (a claim Watson would have loved, of
course).
Later theorists such as Dulany
and Spielberger and DeNike challenged those studies. They
pointed
out a number of potential problems. One, for example, was that whatever
people might have thought was going on would have been implicitly
disconfirmed
once extinction started, since they would now be collecting evidence against
their hypothesis. Another was that a number of correlated hypotheses
could have increased responding, but that these weren't included by the
earlier experimenters. For instance, if you suspect you're being
reinforced
for mentioning species of dogs, then you may say "chihuahuas, collies,
daschunds, terriers, pugs," etc. Note that these are plurals. But when
the experimenter asks you what you believed the purpose of the
experiment
involved, you report that you were being stroked for coming out with
dogs,
which gets you coded (unfairly) as having shown conditioning without
awareness.
And finally, Dulany demonstrated that the people who showed verbal
conditioning
were those who at the time had a correlated hypothesis (i.e.,
were
aware that something was going on, and had a theory that would result
in
increasing responses that the experimenter would count as correct by
administering
reinforcement).
I think a true Skinnerian
wouldn't have been much bothered by Dulany's or Spielberger and
DeNike's
results. Awareness for them could be defined operationally as a
series of answers to questions on a survey (much as Watson defined
emotions
as nothing more than certain behaviors like crying or shaking). Those
answers
constitute verbal behavior, as well. All a true Skinnerian need do in
this
situation is talk about the conditions under which one type of
verbal
behavior (performance on a survey) accompanies another
(conditioning).
I include this example because I want to give you a flavor of the
extraordinarily
different ways in which people interpret scientific research. To go
back
to the work by the philosopher Thomas Kuhn (mentioned
in
Chapter 1), Skinner's approach represents a completely different
paradigm.
And people in different paradigms can only rarely have useful
discussions
with one another about the foundational and philosophical assumptions
that
make science and the world meaningful for them. It is a bit like
arguing
religious beliefs.
B. Tolman's Expectancy Approach
Skinner's first major
publication,
The behavior of organisms, appeared in 1938. To give you a time
frame, Tolman published one of his major works, Purposive behavior
in
animals and men, in 1932. At the time, behaviorism was the only
game
in town. Tolman regarded himself as a behaviorist, but he was like no
other
theorist then around. As you may gather from the title of his book, he
believed that behavior was guided by an organism's purposes and goals.
Thus, his brand of behaviorism is called purposive behaviorism.
Tolman was a theorist, in
contrast to Skinner. He build models around the notion of intervening
variables that came between a stimulus and a response. These
variables
in large part involved cognitions: beliefs, expectancies,
desires, and knowledge. The argument that he and others
who
have used intervening variables make is that theories with such
variables
are more successful in their predictions than those without. He didn't
worry about Watson's dictum that private events were illegitimate in a
science of psychology, since the proof of the legitimacy of the concept
for Tolman was its track record. Tolman was the precursor of people
like
Bandura who built models around observational learning, and more
generally, of the cognitive revolution that occurred in
American
psychology in the 1960s.
Several cognitions were
particularly
important in Tolman's work. One of these we have already met: the
notion
of a cognitive map. According to Tolman, animals observing,
exploring,
and experiencing their environments would come to have representations
of the lay-out of those environments. Thus, he performed experiments in
which he showed that animals would illustrate they had learned an
environment
once properly motivated to do so (e.g., the Tolman and Honzik
experiment
on latent learning discussed earlier), and that they knew how
to
get around obstacles and take novel shortcuts, when their normal routes
were no longer available. We can discuss such learning in terms of
connections
of stimulus (S-S) associations, but it was clearly observational
learning,
in many of Tolman's studies.
Another cognition that
was
quite important (and that prefigured many modern theories of learning)
was the notion of an expectancy or expectation: a
belief
that some event ought to occur in some situation based on past
experiences.
In discussing the situations found in instrumental and classical
conditioning,
Tolman provided examples of two types of expectancies. One may be
written
as follows:
Ej:
S1 -----> S2
This may be read as stating the content of expectancy Ej:
That
expectancy tells us that when S1 occurs, S2
may be expected to follow. If we substitute the CS for S1
and the UCS for S2, we see that we obtain a
situation
corresponding to classical conditioning. But this situation extends far
beyond classical conditioning. It may also explain how we build
cognitive
maps: we learn that this part of the route is normally succeeded by
this
other part.
As for instrumental
conditioning,
the expectancies may be given as below:
Ek:
S3 Ra -----> S4
El:
S3 Rb -----> S5
These two expectancies, Ek and El
(the subscripts are just to keep them separate; we have a huge number
of
expectancies in which these stimuli are specified rather than indicated
through abstract mathematical variables), basically state that in the
presence
of stimulus S3, one response (Ra)
will
lead to stimulus S4, and the other response (Rb)
will result in a different stimulus. If you view these latter stimuli
as
rewards or punishers, then you obtain the expectancies that account for
approach or avoidance.
We can now add the
notions
of value and valence to account for what an animal will
do.
The value of an expectancy has to do with the strength of its terminal
stimulus. If we temporarily regress to speaking of reinforcers and
punishers,
there are strong and weak reinforcers, just as there are strong and
weak
punishers. As you might expect, the strong ones are of greater
motivational
value than the weak ones. As for whether something is positive or
negative,
this involves the notion of its valence or sign.
Given this, we can now
state
some simple rules for what an animal will do in any given situation.
One
is that given a choice between two positive valence responses, the
animal
will choose the stronger. As an example, consider the following
expectancies
in an experiment with monkeys:
Ek:
Tone - Lift White Cup -----> find banana chip
El:
Tone - Lift Blue Cup -----> find piece of lettuce
Here, we presume the animal is faced with a choice involving two
down-turned
cups. Each has a reinforcer hidden beneath it, and the animal may
choose
the reinforcer underneath one of the cups. From past experience, it has
learned that the white cup hides a banana chip, and the blue cup hides
a lettuce leaf. Banana chips are stronger reinforcers: They are
high-value
positive-valence outcomes. Thus, our principle states the animal ought
to choose the white cup. In common words, choose the better of two
goods.
Our second rule will
involve
negative valences. We have a rat in a chamber that may leave by one of
two doors. It is being shocked in the chamber, so there is every reason
to leave. If it goes through the north door, the shock reduces by half,
and if it goes through the south door, the shock reduces by a fourth.
In
this case, the principle is choose the weaker of two
negative-valence
outcomes. Or in plain English, if you have to, go for the lesser of
two evils.
That gives you a bit of a
taste of Tolman's theory. We will talk about several more relevant
studies
later. Tolman and Hull (see the next section), in particular,
constantly
chased one another's experiments and theories, arguing about whether
the
results suggested the need for cognitive factors or not. Interestingly
enough, they both utilized intervening variables, although Hull's
system
was far more developed and organized than Tolman's. But Tolman had a
gift
for finding the weakness in one of Hull's claims, and doing an
experiment
that would seem to demonstrate a result the exact opposite of what Hull
predicted. That in part was the case with the latent learning
study,
since Hull's model allows formation of an association only with a very
special type of reinforcing event called a drive reduction. But
in latent learning, no such event occurred on the first 10 trials for
the
group running without a reinforcer.
It's hard to imagine two
approaches more different, and yet in some respects similar, than
Tolman's
and Skinner's. They were both concerned with large-scale behavior
rather
than the minute muscle movements of Hull's system. But Tolman freely
speculated
on intervening variables while Skinner loathed them. One placed the
cause
of behavior squarely within a cognitive or representation-level
approach,
but the other kept as close to a behavioral-level approach as was
possible.
And the specific details of each theorist's approach were generally
ignored
by most people, although each had enormous influence on subsequent work
or theoretical approaches. Indeed, one of Tolman's students, Krechevsky,
developed the notion that animals during learning test hypotheses
about which stimulus element they are supposed to notice. As we will
see
in a later chapter, this notion evolved into modern-day attentional
theories
of discrimination learning.
C. A Note On The Interrelationship Between Classical &
Operant Conditioning
It should by now be obvious
that there are many close interrelationships between classical and
instrumental
conditioning, Skinner's distinction between the two not withstanding.
In
some cases, they are difficult to tell apart (as in the work on learned
taste aversions, which may be analyzed from either a classical or an
instrumental
conditioning perspective). In others, they are clearly intertwined.
Secondary
reinforcers and aspects of chaining clearly involve classical
conditioning,
and classical conditioning has sometimes been used to account for what
happens in avoidance learning (see the discussion of Mowrer's
two-factor
theory of avoidance learning in the next chapter). So, how
different
are they, really?
Several theorists have
tried
to answer this question from different perspectives. One approach
simply
attempts to cut the Gordian knot by applying similar models to each. In
a later chapter in which we examine theories of discrimination
learning,
we will come across attentional and rehearsal concepts that will remind
you of the corresponding models in classical conditioning. There have
been
some attempts, for example, to modify the Rescorla-Wagner model
to handle the strength of instrumental learning by treating the S
and the R as CSs in compound conditioning, and the RF
as the UCS (see, for example, Wasserman, Elek, Chatlosh,
and
Baker). We have already seen some of the predictions regarding
blocking
and overshadowing that would naturally arise from application of such a
model.
Another approach is more
direct: If Skinner is correct in identifying these two types of
learning
as really involving different types of muscle systems (voluntary versus
involuntary muscles), then we ought not to be able to instrumentally
condition
involuntary reflexes. However, there is now quite a lot of work in the
area of biofeedback on different species (including humans)
that
demonstrates modifying involuntary responses through operation of
instrumental
reinforcers is feasible (see, for example, Miller).
Whether the same models
ultimately
apply to these two areas or not, do note that there will always be the
other type of learning occurring whenever you train instrumental or
classical
conditioning. Reinforcers and punishers are also significant biological
events that act as UCSs, so that instrumental conditioning using
outcomes
should generally include some aspects of classical conditioning.
Similarly,
biologically significant UCSs in classical conditioning may act as
outcomes
influencing the responses the animal makes prior to their presentation.
Learning in the real
world
doesn't always occur in neat packets that allow one type of association
to form, and not another.
Partial Bibliography
Amsel, A. (1958). The role of frustrative nonreward in noncontinuous
reward
situations. Psychological Bulletin, 55, 102-119.
Allison, J. (1989). The nature of reinforcement. In S.B. Klein &
R.R. Mowrer (Eds.), Contemporary learning theories: Instrumental
conditional
theory and the impact of biological constraints on learning
(13-39).
NJ: Erlbaum.
Badia, P., & Culbertson, S. (1972). The relative aversiveness of
signalled vs. unsignalled escapable and inescapable shock. Journal
of
the Experimental Analysis of Behavior, 17, 463-471.
Badia, P., Culbertson, S., & Harsh, J. (1973). Choice of longer
or stronger signalled shock vs. shorter or weaker unsignalled shock. Journal
of the Experimental Analysis of Behavior, 19, 25-32.
Bandura, A. (1965). Influence of models' reinforcement contingencies
on the acquisition of imitative responses. Journal of Personality
and
Social Psychology, 1, 589-595.
Boe, E.E., & Church, R.M. (1967). Permanent effects of
punishment
during extinction. Journal of Comparative and Physiological
Psychology,
63, 486-492.
Bolles, R.C. (1970). Species-specific defense reactions and
avoidance
learning. Psychological Review, 77, 32-48.
Breland, K., & Breland, M. (1961) The misbehavior of organisms.
American psychologist, 16, 681-684.
Brown, J.S., Martin,R.C., & Morrow, M.W. (1964). Self-punitive
behavior
in the rat: Facilitative effects of punishment on resistance to
extinction.
Journal of Comparative and Physiological Psychology, 57, 127-133.
Brown, Pl., & Jenkins, H.M. (1968). Auto-shaping of
the pigeon's key peck. Journal
of the Experimental Analysis of Behavior, 11, 1-8.
Butler, R.A. (1953). Discrimination learning by rhesus monkeys to
visual
exploration motive. Journal of Comparative and Physiological
Psychology,
46, 95-98.
Campbell, P.E., Batsche, C.J., & Batsche, G.M. (1972).
Spaced-trials
reward magnitude effects in the rat: Single versus multiple food
pellets.
Journal of Comparative and Physiological Psychology, 81,
360-364.
Capaldi, E.J. (1978). Effects of schedule and delay of reinforcement
on acquisition speed. Animal Learning and Behavior, 6, 330-334.
Colwill, R.M., & Rescorla, R.A. (1985). Postconditioning
devaluation
of a reinforcer affects instrumental responding. Journal of
Experimental
Psychology: Animal Behavior Processes, 11, 120-132.
Crespi, L.P. (1942). Quantitative variation in incentive and
performance
in the white rat. American Journal of Psychology, 55,
467-517.
Daly, H.B. (1974). Reinforcing properties of escape from frustration
aroused in various learning situations. In G.H. Bower (Ed.), The
psychology
of learning and motivation (Vol 8, 187-231). NY: Academic.
D'Amato, M.R. (1970). Experimental psychology: Methodology,
psychophysics,
and learning. NY: McGraw-Hill.
D'Amato, M.R., Sarafin, W.R., & Salmon, D. Long-delay
conditioning
and instrumental learning: Some new findings. In N.E. Spear and R.R.
Miller
(Eds.), Information processing in animals: Memory mechanisms
(113-142).
NJ: Erlsbaum.
Dember, W.N., & Fowler, H. (1958). Spontaneous alternation
behavior.
Psychological Bulletin, 55, 412-428.
Dollard, J.C., & Miller, N.E. (1950). Personality and
psychotherapy.
NY: McGraw-Hill.
Dulany, D. E. (1968). Awareness, rules, and propositional control: A
confrontation with S-R behavior theory. In T.R. Dixon & D.C. Horton
(Eds.), Verbal behavior and general behavior theory. NJ:
Prentice-Hall.
Dwyer, D.M., Mackintosh, N.J., & Boakes, R.A. (1998).
Simulatneous
activation of the representations of absent cues results in the
formation
of an excitatory association between them. Journal of Experimental
Psychology:
Animal Behavior Processes, 24, 163-171.
Egger, M.D., & Miller, M.E. (1963). When is a reward
reinforcing?
An experimental study of the information hypothesis. Journal of
Comparative
and Physiological Psychology, 56, 132-137.
Ferster, C.B., & Skinner, B.F. (1957). Schedules of
reinforcement.
NY: Appleton-Century-Crofts.
Flaherty, C.F. (1982). Incentive contrast: A review of behavioral
changes
following shifts in reward. Animal Learning and Behavior, 10,
409-440.
Fowler, H., & Miller, N.E. (1963). Facilitation and inhibition
of
runway performance by hind- and forepaw shock of various intensities. Journal
of Comparative and Physiological Psychology, 56, 801-806.
Fowler, H., & Trapold, M.A. (1962). Escape performance as a
function
of delay of reinforcement. Journal of Experimental Psychology, 63,
464-467.
Garcia, J., Ervin, F.R., & Koelling, R.A. (1966). Learning with
prolonged delay of reinforcement. Psychonomic Science, 5,
121-122.
Greenspoon, J. (1955). The reinforcing effect of two spoken sounds
on
the frequency of two responses. American Journal of Psychology, 68,
409-416.
Grice, G.R. (1948). The relation of secondary reinforcement to
delayed
reward in visual discrimination learning. Journal of Experimental
Psychology,
38, 1-16.
Guthrie, E.R. (1952 ). The psychology of learning. (Revised
edition)
NY: Harper & Row.
Guttman, N., & Kalish, H.I. (1956). Discriminability and
stimulus
generalization. Journal of Experimental Psychology, 51, 79-88.
Hammond, L.J. (1980). The effect of contingency upon the appetitive
conditioning of free operant behavior. Journal of the Experimental
Analysis
of Behavior, 34, 297-304.
Hanson, H.M. (1959). Effects of discrimination training on stimulus
generalization. Journal of Experimental Psychology, 58,
321-334.
Herrnstein, R.J. (1970). On the law of effect. Journal of the
Experimental
Analysis of Behavior, 13, 243-266.
Jenkins, H.M., & Moore, B.R. (1973). The form of the
autoshaped response with food or water reinforcers. Journal of the Experimental Analysis of
Behavior, 20, 163-181.
Killeen. P. (1978). Superstition: A matter of bias, not
detectability.
Science, 199, 88-90.
Kohn, B., & Dennis, M. (1972). Observation and discrimination
learning
in the rat: Specific and nonspecific effects. Journal of
Comparative
and Physiological Psychology, 78, 292-296.
Kraeling, D. (1961). Analysis of amount of reward as a variable in
learning.
Journal of Comparative and Physiological Psychology, 54,
560-565.
Krechevsky, I. (1932). "Hypotheses" in rats. Psychological
Review,
39, 516-532.
Kuhn, T.S. (1970). The structure of scientific revolutions
(second
edition). Chicago: University of Chicago Press.
Lawrence, D.H., & DeRivera. J. (1954). Evidence for relational
transposition.
Journal of Comparative and Physiological Psychology, 47,
465-471.
Lieberman, D.A., Davidson, F.H., & Thomas, G.V. (1985). Marking
in pigeons: The role of memory in delayed reinforcement. Journal of
Experimental Psychology: Animal Behavior Processes, 11, 611-624.
Macfarlane, D.A. (1930). The role of kinesthesis in maze learning. California
University Publication Psychology, 4, 277-305.
MacPhail. E.M. (1968). Avoidance responding in pigeons. Journal
of
the Experimental Analysis of Behavior, 11, 625-632.
McNamara, H.J., Long, J.B., & Wike, F.L. (1956). Learning
without
response under two conditions of external cues. Journal of
Comparative
and Physiological Psychology, 49, .
Melton, A.W., & Irwin, J.M. (1940). The influence of degree of
interpolated
learning on retroactive inhibition and overt transfer of specific
responses.
American Journal of Psychology, 53, 173-203.
Menzel, E.W. (1978). Cognitive mapping in chimpanzees. In S.H.
Hulse,
H. Fowler, & W.K. Honig (Eds.), Cognitive processes in animal
behavior
(375-422). NJ: Erlbaum.
Miller, N. E. (1978). Biofeedback and visceral learning. Annual
Review
of Psychology, 29, 373-404.
Morris, R.G.M., Garrud, P., Rawlins, J.N.P., & O'Keefe, W.
(1982).
Olton, D.S. (1978). Characteristics of spatial memory. In S.H.
Hulse,
H. Fowler, & W.K. Honig (Eds.), Cognitive processes in animal
behavior
(341-373). NJ: Erlbaum.
Postman, L. (1974). Transfer, interference, and forgetting. In J.W.
Kling and L.A. Riggs (Eds.), Experimental psychology. NY: Holt,
Rinehart, & Winston.
Premack, D. (1959). Toward empirical behavior laws: I. Positive
reinforcement.
Psychological Review, 66, 219-233.
Ratliff, R.G., & Ratliff, A.R. (1971). Runway acquisition and
extinction
as a joint function of magnitude of reward and percentage of rewarded
acquisition
trials. Learning and Motivation, 2, 289-295.
Rescorla, R.A. (1997). Response-inhibition in extinction. Quarterly
Journal of Experimental Psychology. B. Comparative and Physiological
Psychology,
50B, 238-252.
Reynolds, G.S. (1961). Attention in the pigeon. Journal of the
Experimental
Analysis of Behavior, 4, 57-71.
Roberts, W.A. (1969). Resistance to extinction following partial and
consistent reinforcement with varying magnitudes of reward. Journal
of Comparative and Physiological Psychology, 67, 395-400.
Seligman, M.E.P. (1970). On the generality of laws of learning. Psychological
Review, 77, 406-418.
Seligman, M.E.P., & Maier, S.F. (1967). Failure to escape
traumatic
shock. Journal of Experimental Psychology, 74, 1-9.
Seward, J.P., & Levy, N. (1949). Sign learning as a factor in
extinction.
Journal of Experimental Psychology, 39, 660-668.
Sheffield, F.D. (1965). Relation between classical conditioning and
instrumental learning. In W.F. Prokasy (Ed.), Classical
conditioning:
A symposium (302-322). NY: Appleton-Century-Crofts.
Shettleworth, S.J. (1975). Reinforcement and the organization of
behavior
in golden hamsters: Hunger, environment, and food reinforcement. Journal
of Experimental Psychology: Animal Behavior Processes, 1, 56-87.
Skinner, B.F. (1938). The behavior of organisms: An experimental
analysis. NY: Appleton-Century-Crofts.
Skinner, B.F. (1964). Behaviorism at fifty. In T.W. Wann (Ed.), Behaviorism
and phenomenology: Contrasting bases for modern psychology
(79-108).
Chicago: U. Chicago Press.
Spence, K.W. (1947). The role of secondary reinforcement in delayed
reward learning. Psychological Review, 54, 1-8.
Spielberger, L.D., & DeNike, L. (1966). Descriptive behaviorism
versus cognitive theory in verbal operant conditioning. Psychological
Review, 73, 306-326.
Staddon, J.E.R., & Simmelhag, V.L. (1971). The "superstition"
experiment:
A reexamination of its implications for the principles of adaptive
behavior.
Psychological Review, 78, 3-43.
The Rocky Horror Show. (1975). Original Australian Cast
Album.
Elephant Records. (Yep: It's better Rock & Roll than the later
American
album based on the movie version, in my opinion...)
Thomas, G. (1981). Contiguity, reinforcement rate, and the law of
effect.
Quarterly Journal of Experimental Psychology, 33B, 33-43.
Thomas, D.R., Mariner, R.W., & Sherry, G. (1969). Role of
pre-experimental
experience in the development of stimulus control. Journal of
Experimental
Psychology, 79, 375-376.
Thorndike, E.L. (1898). Animal intelligence: An experimental study
of
the associative processes in animals. Psychological Review
Monograph
Supplements 2, no. 8.
Thorndike, E.L. (1913).The psychology of learning. NY:
Columbia University.
Tolman, E. C. (1932). Purposive behavior in animals and men.
NY: Century.
Tolman, E.C., & Honzik, C.H. (1930). Introduction and removal of
reward and maze performance in rats. University of California
Publications
in Psychology, 4, 257-275.
Trapold, M.A., & Fowler, H. (1960). Instrumental escape
performance
as a function of the intensity of noxious stimulation. Journal of
Experimental
Psychology, 60, 323-326.
Verplanck, W.S. (1955). The operant, from rat to man.
Transactions
of the New York Academy of Sciences, Series 11, 17 (8),
594-601.
Wagner, A.R., Logan, F.A., Haberlandt, K., & Price, T. (1968).
Stimulus
selection in animal discrimination learning. Journal of
Experimental
Psychology, 76, 171-180.
Wasserman, E.A., Elek, S.M., Chatlosh, D.C., & Baker, A.G.
(1993).
Rating causal relations: Role of probability in judgments of
response-outcome
contingency. Journal of Experimental Psychology: Learning, Memory,
and
Cognition, 19, 174-188.
Watson, J.B. (1913). Psychology as the behaviorist views it. Psychological
Review, 20, 158-177.
Watson, J.B. (1926 Excerpts from "What the nursery has to say about
instincts." In C. Murchison (Ed.), Psychologies of 1925. NY:
Clark
U. Press.
Watson, J.B. (1930). Behaviorism. NY: Norton.
Watson, J.B., & Rayner, R. (1920). Conditioned emotional
reactions.
Journal of Experimental Psychology, 3, 1-14.
Watson, J.S. (1967). Memory and "contingency analysis" in infant
learning.
Merrill-Palmer Quarterly, 12, 55-76.
Some Relevant Internet Sites (but there are many more out
there!):
Animal
Cognition Page:
(http://www.pigeon.psy.tufts.edu/psych26/history.htm)
(note particularly the links to Thorndike, Tolman, and
Skinner's
stuff. There's a graph of Thorndike's results with puzzle-box learning
that you should look at.)
The
Behaviorist Manifesto:
(http://www.yorku.ca/dept/psych/classics/Watson/views.htm)
(Watson's classic paper)
Emotional
Conditioning:
( http://www.yorku.ca/dept/psych/classics/Watson/emotion.htm)
(The Watson & Rayner paper reporting on their study
with
Little Albert)
Cognitive
Maps:
(http://www.yorku.ca/dept/psych/classics/Tolman/Maps/maps.htm)
(A paper by Tolman reviewing some of the cognitive map
studies)
(Note: The three papers above are from the Classics in the
History
of Psychology webpage; you have a link for it in Chapter 1)
1. Chapter © 1998 by Claude G. Cech
 Several features of these results should be noted. First, the stimulus
that received the most pecks for each group was S+:
That
is where the peak of each generalization gradient may be found.
(As you will see later, this need not always be the case. Certain
experiences
such as discrimination training may alter the peak and shape of a
generalization
gradient.) Second, there was a relatively smooth drop-off of
responding
as the wavelengths of the stimuli increasingly differed from the S+.
And finally, the curves were symmetric: The left-hand side of
each
curve looked approximately like the right-hand side.
Several features of these results should be noted. First, the stimulus
that received the most pecks for each group was S+:
That
is where the peak of each generalization gradient may be found.
(As you will see later, this need not always be the case. Certain
experiences
such as discrimination training may alter the peak and shape of a
generalization
gradient.) Second, there was a relatively smooth drop-off of
responding
as the wavelengths of the stimuli increasingly differed from the S+.
And finally, the curves were symmetric: The left-hand side of
each
curve looked approximately like the right-hand side.
 representing
the conflict point.
representing
the conflict point.
 It is important to note that all groups received the same amount of
learning in Phase 2 (in terms of number of trials and what the
reinforcer
was). Thus, we might have expected each group to display the same
relative improvement. But, that did not happen. Because these contrasts
occurred during a post-acquisition period involving identical
additional
training, they are generally interpreted as demonstrating an effect not
on learning (or acquisition), but rather on performance.
That argument is particularly compelling for Group 1: They continued to
receive additional reinforced training during Phase 2, yet they
apparently
got worse! Contrasts of this sort are termed incentive contrasts.
It is important to note that all groups received the same amount of
learning in Phase 2 (in terms of number of trials and what the
reinforcer
was). Thus, we might have expected each group to display the same
relative improvement. But, that did not happen. Because these contrasts
occurred during a post-acquisition period involving identical
additional
training, they are generally interpreted as demonstrating an effect not
on learning (or acquisition), but rather on performance.
That argument is particularly compelling for Group 1: They continued to
receive additional reinforced training during Phase 2, yet they
apparently
got worse! Contrasts of this sort are termed incentive contrasts.